-
1.1《药学英语》
-
1.2总 序
-
1.3前 言
-
1.4目录
-
1.5Unit 1
-
1.5.1Text Order of the President of the People’s Republ...
-
1.5.2Supplementary Reading DRUG ADMINISTRATION LAW OF T...
-
1.6Unit 2
-
1.6.1Text A General Introduction to OTC Drugs
-
1.6.2Supplementary Reading Reading a Drug Label
-
1.7Unit 3
-
1.7.1Text Package Insert:LEXINOR 100 mg
-
1.7.2Supplementary Reading Neptunlong
-
1.8Unit 4
-
1.8.1Text Conversations Often Taking Place in a Pharmac...
-
1.8.2Supplementary Reading Making an Appointment with a...
-
1.9Unit 5
-
1.9.1Text BP 2007 Cefalexin Monohydrate(Ph Eur monograp...
-
1.9.2Supplementary Reading BP 2007 Amantadine Hydrochlo...
-
1.10Unit 6
-
1.10.1Text USP 30 Acyclovir
-
1.10.2Supplementary Reading Folic Acid
-
1.11Unit 7
-
1.11.1Text A Brief Introduction to Traditional Chinese M...
-
1.11.2Supplementary Reading Acupuncture
-
1.12Unit 8
-
1.12.1Text Importance of Influenza Vaccination for Healt...
-
1.12.2Supplementary Reading Types of Vaccines
-
1.13Unit 9
-
1.13.1Text Organization and Personnel in USA GMP
-
1.13.2Supplementary Reading Subpart G-Packaging and Labe...
-
1.14Unit 10
-
1.14.1Text Farm-to-Table Initiatives for Safer Domestic,...
-
1.14.2Supplementary Reading Sampling Produce for Pathoge...
-
1.15附录1 《中华人民共和国药品管理法》(中文版)
-
1.16附录2 《中华人民共和国药品管理法》(英文版)
-
1.17附录3 《药品说明书和标签管理规定》
-
1.18附录4 《血液制品管理条例》
-
1.19附录5 《生物制品批签发管理办法》
-
1.20附录6 《疫苗流通和预防接种管理条例》
-
1.21附录7 常用医保药品通用名、商品名、英文名对照表
-
1.22Vocabulary
-
1.23汉英词汇总表
-
1.24Key to the Exercises and Scripts
Key to the Exercises and Scripts
Unit 1
Exercises
Task 1
1.B 2.A 3.C 4.A 5.C
Task 2
第七条 开办药品生产企业,须经企业所在地省、自治区、直辖市人民政府药品监督管理部门批准并发给《药品生产许可证》,凭《药品生产许可证》到工商行政管理部门办理登记注册。无《药品生产许可证》的,不得生产药品。《药品生产许可证》应当标明有效期和生产范围,到期重新审查发证。药品监督管理部门批准开办药品生产企业,除依据本法第八条规定的条件外,还应当符合国家制定的药品行业发展规划和产业政策,防止重复建设。
Notes to Supplementary Reading
1.Article 14 The establishment of a drug wholesaler shall be subject to approval of the local drug regulatory department of the people’s government of the province, autonomous region or municipality ...and be granted the Drug Supply Certificate
the establishment of a drug wholesaler:药品批发企业
be subject to approval:经过……批准
the local drug regulatory department:地方药品监督管理部门
be granted the Drug Supply Certificate:被发给《药品经营许可证》
全句可译为:第十四条 开办药品批发企业,需经企业所在地省、自治区、直辖市人民政府药品监督管理部门的批准并发给《药品经营许可证》。
2.the establishment of a drug retailer shall be subject to approval and be granted the said certificate by the local drug regulatory department at or above the county level
the establishment of a drug retailer:药品零售企业
be granted the said certificate:被发给上述证明(即《药品经营许可证》)
at or above the county level:在县级以上
the local drug regulatory department at or above the county level:当地县级以上的药品监督管理部门
3.The valid term and the scope of business shall be indicated in the Drug Supply Certificate.For renewal of the certificate upon expiration, reexamination is required.
the valid term and the scope of business:有效期和经营范围
for renewal of the certificate:证书的更新,重新发证
upon expiration:有效期满,到期
全句可译为:《药品经营许可证》应标明有效期和经营范围,到期重新审核发证。
4.When giving approval to the establishment of a new distributor, the drug regulatory department shall see to it that, apart from the requirements specified in Article 15 of this Law that should be met, the principles of appropriate location and convenient purchase of drugs by the people are adhered to.
see to it that:注意,务必做到,保证
apart from the requirements specified in Article 15 of this Law:除了本法第十五条规定的必要条件外
the principles of appropriate location and convenient purchase of drugs by the people:合理布局和方便群众购药的原则
be adhered to:遵守,遵循
全句可译为:在批准开办新的药品经营企业时,除了依据本法第十五条规定的条件外,还应遵循合理布局和方便群众购药的原则。(原文:药品监督管理部门批准开办药品经营企业,除了依据本法第十五条的条件外,还应遵循合理布局和方便群众购药的原则。)
5.Article 16 Drug distributors shall conduct business according to the Good Supply Practice for Pharmaceutical Products (GSP)formulated by the drug regulatory department under the State Council on the basis of this Law.
conduct business:经营(药品)生意
GSP = Good Supply Practice,我国定型翻译成“药品经营质量管理规范”
on the basis of this Law:以本法为依据
全句可译为:第十六条 药品经营企业必须按照国务院药品监督管理部门依据本法制定的《药品经营质量管理规范》经营药品。
6.The specific measures and schedule for implementing the GSP shall be formulated by the drug regulatory department under the State Council.
the specific measures and schedule:具体措施和安排
be formulated:由……(做出)规定
全句可译为:《药品经营质量管理规范》的具体实施办法、实施步骤由国务院药品监督管理部门规定。
7.In the record shall be indicated the adopted name in China, dosage form, strength or size, batch number, date of expiry, manufacturer, purchase (or sale)unit, amount of the drug purchased (or sold), purchase or sale price, date of purchase (or sale), and other items specified by the drug regulatory department under the State Council.
adopted name:(药品)通用名。药品通常有3个药名:商品名、通用名及化学名。通用名是药品的法定名称,即收入国家药品标准的名称,如治疗感冒常用的扑热息痛,“对乙酰氨基酚”就是其通用名。但一种药品如果有多家药厂同时生产,有的厂家就会申请给自己的产品起另一个名字,以有别于其他厂家的产品,另起的这个名字就是药品的商品名。例如:扑热息痛、泰诺林、百服宁、必理通等。化学名则为:对羟基苯乙酰胺。药品使用通用名有利于百姓安全用药。
dosage form, strength:剂型与规格(指含量大小)
specified by the drug regulatory department under the State Council是分词短语代替定语从句,修饰other items,相当于other items which are specified by the drug regulatory department under the State Council 由国务院药品监督管理部门规定的其他内容。
全句可译为:购销记录必须注明药品的(中文)通用名称,剂型、规格、批号、有效期、生产商、购(销)货单位、购(销)货数量、购(销)价格、购(销)货日期及国务院药品监督管理部门规定的其他内容。
8.They shall refuse to dispense incompatible or over-dose prescriptions; when necessary, they may do the dispensing only after corrections or re-signing is made by the prescribing physician.
They shall refuse to dispense:他们应当拒绝调配(药品)。由于本条是对药品经营企业在调配处方时的规定,因此句中they指的是企业(药店),而不是员工。
incompatible or over-dose prescriptions:有配伍禁忌或超剂量的处方
corrections or re-signing:修改或重新签字
全句可译为:对有配伍禁忌或者超剂量的处方,应当拒绝调配;必要时经处方医师更正或重新签字,方可调配。
9.placing drugs in and releasing them from storage:药品的入库和出库
Comprehension Questions
1.The establishment of a drug wholesaler shall be subject to approval of the local drug regulatory department of the people’s government of the province, autonomous region or municipality directly under the Central Government and be granted the Drug Supply Certificate.
2.The valid term and the scope of business shall be indicated in the Drug Supply Certificate.
3.A drug distributor to be established shall meet the following requirements:
(1)having legally qualified pharmaceutical professionals;
(2)having the business operation premises, equipment, warehouses and hygienic environment required for drug distribution;
(3)having the units or personnel for quality control over the drugs to be distributed; and
(4)having rules and regulations to ensure the quality of the drugs to be distributed.
4.Drug distributors shall conduct business according to the Good Supply Practice for Pharmaceutical Products (GSP)formulated by the drug regulatory department under the State Council.
5.Drug distributors shall sell drugs properly and make correct description of usage, dosage and cautions; prescription for dispensing shall be checked, and no drugs listed in the prescription may be changed or substituted without authorization.
6.They shall refuse to dispense incompatible or over-dose prescriptions; when necessary, they may do the dispensing only after corrections or re-signing is made by the prescribing physician.
Drug distributors shall indicate the origin of the Chinese crude drugs to be sold.
7.A drug distributor shall establish and apply a system for drug storage, and take necessary measures to ensure quality, such as cold storage, protection against freeze and humidity and avoidance of insects and rodents.
8.No drugs other than the Chinese crude drugs may be sold at town and country fairs, but drug retailers holding the Drug Supply Certificate may, within the specified business scope, sell such drugs at stores they set up at the fairs.
Unit 2
Exercise
Task 1
1.Over-the-counter (OTC)drug are those drugs that are available to consumers without a prescription.
2.Your doctor writes a prescription that tells the pharmacist what you need.You pick up the medicine at a pharmacy.
3.Such as aspirin and acetaminophen.
4.The U.S.Food and Drug Administration (FDA).
5.FDA also has the authority to decide when a prescription drug is safe enough to be sold directly to consumers over the counter.
6.By the New Drug Application (NDA)process.
7.More than 700 products.
8.Increased access to OTC medicines is especially important for maturing population.
9.Two out of three older Americans rate their health as excellent to good.
10.The need grows to become better informed about self-care.
Task 2
当FDA考虑重新分类处方药为非处方药时,安全是主要问题。大部分的非处方药,不像保健食品、膳食补充品(包括中草药)及辅助疗法,已经被科学广泛地研究。然而,所有的药品都有其益处和风险,而且人们为了获得药效不得不承担一定的风险。定义一种可接受危险度需要有判断力。
Notes to Supplementary Reading
1.Drug Label:药品标签
2.Nonprescription drugs are required to have labels that explain what a drug’s benefits and risks are and how to use the drug correctly.
该句为复合句。that explain what a drug’s benefits and risks are and how to use the drug correctly 为定语从句修饰labels。其中what a drug’s benefits and risks are名词性从句,及how+ to use the drug correctly不定式短语同时做定语从句中explain的两个宾语。
全句可译为:非处方药要求贴有药品标签,该标签对药品的益处和风险,以及如何正确使用药品做了说明。
3.The label is entitled “Drug Facts”.可译为:该标签称为“药品说明”。
4.The same generic drug may be sold under several different trade (brand)names.
全句可译为:同一种通用名的药可能以不同的商品名出售。
5.Symptoms or disorders for which the drug product is recommended are listed.
该句主干:Symptoms or disorders are listed.其中for which the drug product is recommended 为限定性定语从句修饰主语symptoms or disorders。
全句可译为:列出药品适用的症状或病症(药品的适应证)。
6.“Ask a doctor before use if you have” lists conditions that can make taking the drug more problematic or unsafe.
全句可译为:“如果你身边有个医生就咨询他”这句话说明服用药品可能存在问题或不安全。
7.The last section lists special warnings for women who are pregnant or breastfeeding and for children, with instructions about what to do in case of an overdose.
全句可译为:最后一项列明对怀孕或哺乳期妇女和小孩的特别警告,及介绍万一用药过量的处理方法。
8.In addition to the drug, drug products—the tablets, capsules, or other formulations that consumers buy—contain substances added to facilitate the administration of the drug, such as ingredients that provide bulk or a pleasant taste and color.
本句的主要成分drug products contain substances (药品含有物质)。其中the tablets, capsules, or other formulations that consumers buy 是对主语drug products的解释,说明药品包含药片、胶囊或消费者购买的其他配方。added to facilitate the administration of the drug 是过去分词短语做后置定语,代替定语从句which are added to facilitate the administration of the drug,修饰 substances,可译为:方便药品服用的物质。
全句可译为:除了药物,药品——药片、胶囊或消费者购买的其他配方——包含方便药品服用的物质,比如能使之成块(服用)或增加色泽和口感的物质。
Comprehension Questions
1.Nonprescription drugs are required to have drug labels.
2.It explains what a drug’s benefits and risks are and how to use the drug correctly.
3.Active ingredients are listed at the top, followed by uses, warnings, directions, other information and inactive ingredients.
4.Yes, it can.
5.The drug itself is the active ingredient.
6.“Ask a doctor or a pharmacist before use if you are taking” refers to drug-drug interactions.
7.The last section in warnings.
8.The substances use for facilitating the administration of the drug, such as ingredients that provide bulk or a pleasant taste and color.
Unit 3
Exercises
Task 1
1.C 2.C 3.C 4.B 5.B
Task 2
口服西替利嗪后一小时内血药浓度达到峰值,成人血药半衰期约为10小时,6—12岁儿童血药半衰期为6小时,2—6岁儿童血药半衰期为5小时。该数据与尿液药物排泄的半衰期一致。成人与儿童从尿液中排泄的累计药量为所给剂量的三分之二。因此,儿童表现的血药清除速度高于成人。
人们对本品的吸收是非常一致的。
血浆浓度水平和给药剂量成线性关系。
西替利嗪与血浆蛋白结合牢固。
Notes to Supplementary Reading
1.Anti-inflammatory, analgesic and antipyretic.It is an inhibitor of restraining epoxidase that the biological synzyme of prostaglandin can be restrained.
全句可译为:本品具有消炎、镇痛、解热作用。通过抑制环氧酶,进而抑制前列腺素生物合成酶。
2.Following single oral dose of 400 mg Neptunlong, the peak plasma concentrations are reached in about 3 to 4 hours.
single oral dose:一次口服
全句译为:一次口服本品400 mg后,3—4小时血药浓度达到峰值。
3.The plasma concentrations become stable in 4 to 6 days after 10 days repeated administration of 400 mg daily with single or divided doses.
repeated administration:连续服用
single or divided doses:一次或分次口服
全句可译为:连续服用本品10日,每日400 mg,一次或分次口服,4到6天后血药浓度达稳定。
4.The total Neptunlong and the metabolites can be found in urine, and are excreted mainly by kidney.
全句可译为:本品主要经肾脏排泄,尿中可找到原型药及其代谢产物。
5.Individuals suffering from alimentary ulcer, serious hepatic and kidney diseases, who are hyperactive to other non-steroidal anti-inflammatory analgesic, patients suffering from blood disease, children, pregnant women and breast feeding women.
individuals suffering from alimentary ulcer:患有消化道溃疡的患者
who are hyperactive to other non-steroidal anti-inflammatory analgesic:对其他非甾体消炎镇痛药过敏患者
children, pregnant women and breast feeding women:儿童、孕妇及哺乳期妇女
全句可译为:患有消化道溃疡、严重肝肾疾病患者,对其他非甾体消炎镇痛药过敏患者,患有血液疾病的患者,儿童、孕妇及哺乳期妇女禁用。
6.The administration should be reduced or stopped if abnormality is found after long-term administration.
全句可译为:长期服用本品后如发现异常,应减少剂量或停用。
Comprehension Questions
1.The mode of action is inhibition of the synthesis of prostaglandin.
2.Following single oral dose of 400 mg Neptunlong, the peak plasma concentrations are reached in about 3 to 4 hours.It leads to a 50-hour elimination half-life.
3.The total Neptunlong and the metabolites can be found in urine, and are excreted mainly by kidney.
4.Neptunlong is indicated to rheumatic arthritis, rheumatoid arthritis, tetanic spondylitis, periarthritis, cervical shoulder wrist syndrome, gout attack, injury and inflammation and pain after operation.
5.400 mg daily, to be taken orally, single or divided dose after meals.The administration should be continued for over one week or follow the doctor’s advice.The stated dose is less than 600 mg daily.
6.The major side effects are stomach-ache, no appetite, diarrhoea, constipation, nausea, thirst and stomatitis as the most frequently occurring symptoms in alimentary canal, along with vertigo, headache, drowsiness, tinnitus, tic and transient abnormality of liver function.
7.Neptunlong is contraindicated to individuals suffering from alimentary ulcer, serious hepatic and kidney diseases, who are hyperactive to other non-steroidal anti-inflammatory analgesic, patients suffering from blood disease, children, pregnant women and breast feeding women.
8.The administration should be reduced or stopped if abnormality is found after long-term administration.Dosage together with anti-coagulant agent should be prudent.
Unit 4
Exercises
Task 1
1.If you want a diagnosis for you illness, you may go to a clinic, a doctor or a hospital.
2.You can get medication over the counter, at local pharmacy or drugstore.
3.Common, widely used and/or low-dosage medications that are not considered dangerous are readily available to consumers without prescription in the U.S.
4.If you still feel the same after three days, you had better see a doctor.
5.The patient has a pricking and fitful pain.
6.Take Tagamet and Metronidazole after meals; take Motilinum 20 minutes before meals.The most frequently reported adverse reactions are stomach discomfort, dizziness and so on.All side effects disappear after cessation of therapy.
7.He felt stomachache, queasy and sick.Few minutes after breakfast, he brought up all that he had eaten.Then he had severe diarrhea with pain.Now he feels so weak and tired.
8.Smecta possesses a fixing and inhibiting effect for virus, bacteria and their toxins in digestive tract; and coating property on the gastrointestinal mucosa.
9.If the symptoms don’t relieve accompanied by frequently diarrhea, you should go to the hospital to do more treatment, such as fluid therapy to improve his electrolyte disturbances.
10.He suggested he buy some band-aid.
Task 2
当在北美生活居住或旅游参观时,人们总希望自己能健健康康,可以享受旅途的乐趣,开展商务活动或是集中于学习研究。而有时人们会得病,感觉不舒服,甚至出现紧急情况。这时,就必须求得专业人员的帮助。有些药品能缓解疾病症状,这正是人们去药店时想要的。人们应该经常跟药剂师确认,所选购的是正确的非处方药。
以下对话经常发生在药店或药房。
对话1
药剂师:您好,想要买什么药?
顾 客:请给我一包止痛药。
药剂师:布洛芬行吗?它对缓解疼痛效果很好。
顾 客:可以,我以前经常用它。请给我一盒。
药剂师:这就是。
顾 客:有小包装的吗?
药剂师:不好意思,小包装的已经卖完,您能等几天吗?
顾 客:用剩的药该怎么办呢?
药剂师:请把瓶盖旋紧,存放在阴凉干燥的地方。
顾 客:谢谢。
对话2 胃痛(消化不良)
药剂师:您好,小姐,要买什么药?
顾 客:这些天来我一直胃痛,该吃什么药?
药剂师:胃痛多久了?
顾 客:三四天了。
药剂师:饭前还是饭后痛?
顾 客:饭后。
药剂师:是什么样的痛?是刺痛、胀痛或是其他样的疼痛?
顾 客:胀痛,我觉得胃部胀。
药剂师:除了胃痛之外,还有什么其他症状?
顾 客:我经常打嗝。
药剂师:三餐是否吃得太多?经常参加运动吗?
顾 客:是的,我吃得太多。我是办公室职员,没时间参加体育运动。
药剂师: 我估计你的胃痛是因营养不良引起的。您吃得过多而运动太少,这能增加您的肠胃负担,引起腹胀和打嗝。我建议您服用“吗丁啉”片。它能促进消化功能。同时要节食,参加适当的体育运动,这样能减轻您的消化问题。
顾 客:谢谢您的建议,我买这种药。
药剂师:这是口服药,每日3次,每次1片。
顾 客:谢谢。
药剂师:不用谢,再见。
Notes to Supplementary Reading
1.a drug that is carefully regulated by the U.S.government:受到美国政府严格管理的药品
2.Going about getting prescription drugs in the United States is quite a longer process than in China.
全句可译为:在美国购买处方药与在中国买相比,是一个长得多的过程。
3.It is not always easy to make a doctor’s appointment because their offices are often very busy with only few slots open for appointments.
全句可译为:因为医生诊所繁忙,通常只有极为有限的时间可供预约,因此要与医生预约是相当难的。
4.If you are really desperate, you can go to a local minor emergency clinic and see any doctor.
全句可译为:如果你对预约医生感到没希望,可以到当地的小型急症诊所找一个医生看。
5.I’m afraid he is fully booked on Monday and Tuesday.
全句可译为:对不起,他星期一和星期二的日程已安排满了。
6.Sorry, but I have to say he is also occupied on Thursday.
全句可译为:抱歉,他星期四的日程也已安排满了。
7.cough without sputum:干咳
8.Finally, you drop off the doctor’s prescription at your nearby pharmacy.The wait is anywhere from 20 minutes to a whole day.
全句可译为:最后,把医生处方留在附近的药店(等候配药)。等候的时间为20分钟到1天。
9.When you pick up your prescription, the well-educated pharmacist will talk over with great care the details of the drugs and the most effective way for you to use them.
全句可译为:当你前往药店取药时,训练有素的药剂师会向你详细介绍药品的情况及最有效的服用方法。
Comprehension Questions
1.If you want a drug that is carefully regulated by the U.S.government, then you will need a prescription from a doctor, or you will not be able to buy it over the counter.
2.Because doctors’ offices are often very busy with only few slots open for appointments.
3.If a patient feeks really desperate, he can go to a local minor emergency clinic and see any doctor.
4.When you go to your appointment, the doctor will ask you many questions about your illness, such as the duration and severity of your condition.He will check you thoroughly as well before deciding to give you a prescription for drugs.
5.Friday.
6.He has a sore throat.
7.He complained that sometimes he coughed without sputum.
8.The doctor advised the patient to have a gastroscopy first.
9.Most Americans just drop off the prescription and then go home to rest and have another family member pick up the drugs later in the day.
10.A well-educated pharmacist will talk over with great care the details of the drugs and the most effective way for you to use them.
Unit 5
Exercises
Task 1
1.It is a white or almost white, crystalline powder.
2.It is sparingly soluble in water, practically insoluble in alcohol.
3.Dissolve 50 mg in carbon dioxide-free water R and dilute to 10 ml with the same solvent.Its PH is 4.0 to 5.5.
4.Dissdve 50 mg in water R and dilute to 100.0 ml with the same solvent.The absorbance of the solution determined at 300 nm is not greater than 0.05.Dilute 2.0 ml of the solution to 50.0 ml with water R.Examined between 200 nm and 300 nm, the diluted solution shows an absorption maximum at 262 nm.The specific absorbance at this maximum is 220 to 245, calculected with reference to the anbydrous substance.
5.Dissdve 50.0 mg of the substance to be examined in mobile phase A and dilute to 50.0 ml with mobile phase A.
6.Dissolve 10 mg of dimethylformamide R and 10 mg of dimethylacetamide R in mobile phase A and dilute to 10.0 ml with mobile phase A.Dilute 10 mg to 100.0 ml with mobile phase A.
7.size:l=0.10 m, φ=4.6 mm,
stationary phase:spherical octadecylsilyl silica gel for chromatography R (5 mm).
8.resolution:minimum of 2.0 between the peaks due to impurity A and to impurity B in the chromatogram obtained with reference solution (c)and minimum of 1.5 between the peaks due to cefalexin and to cefotaxime in the chromatogram obtained with reference solution (f).
9.Test solution
Dissolve 50.0 mg of the substance to be examined in water R and dilute to 50.0 ml with the same solvent.
Reference solution (a)
Dissolve 50.0 mg of cefalexin monohydrate CRS in water R and dilute to 100.0 ml with the same solvent.
Reference solution (b)
Dissolve 10 mg of cefradine CRS in 20 ml of reference solution (a)and dilute to 100 ml with water R.
10.Column:size:l=0.25 m, φ=4.6 mm,
stationary phase:octadecylsilyl silica gel for chromatography R (5 mm).
Mobile phase:Methanol R, acetonitrile R, a 13.6 g/l solution of potassium dihydrogen phosphate R, water R (2∶5∶10∶83 V/V/V/V).
Task 2
氟喹啉酸
使用薄层色谱仪(依法测定),附录(2.2.27),使适当的硅胶做涂层,配有最佳光密度254纳米的荧光指示器。
供试品溶液(的配制)
取供试品50毫克,溶于稀氨水R1,并用相同溶剂稀释至5毫升。
对照溶液(的配制)
取化学参比物质氟喹啉酸10毫克,溶于01.毫升稀氨水R1与90毫升水R,并用水R稀释至100毫升。取2毫升配制好的溶液用水R稀释至10毫升。
向薄层板点加两种溶液各5毫升,在色谱层析槽的底部放置一个装有50毫升浓氨水的蒸发皿,盖上层析槽盖,将薄层板暴露在氨蒸汽中15分钟。取出薄层板移至另外的层析槽,使用10份乙腈R,20份浓氨水R,40份甲醇R与40份二氯甲烷R的混合溶液中,在15厘米的宽度上展开。将薄层板在空气中晾干,在254纳米(波长)的紫外光下检测。由供试品溶液获得的氟喹啉酸在色谱图上的斑点颜色不得深于对照溶液的斑点颜色(0.2%)
Notes to Supplementary Reading
1.Definition
Amantadine hydrochloride contains not less than 98.5 percent and not more than the equivalent of 101.0 percent of tricyclo[3.3.1.13,7]decan-1-amine, calculated with reference to the anhydrous substance.
全句可译为:定义
按干燥品计算,本品盐酸金刚烷胺含三环[3.3.1.13,7] 癸烷-1-胺为标示量的98.5%—101.0%之间。
2.Characters
A white or almost white, crystalline powder, freely soluble in water and in alcohol, practically insoluble in ether.It sublimes on being heated.
freely soluble in water and in alcohol相当于:one part of solute soluble in one part to 10 parts of water or alcohol.
全句可译为:性状
本品为白色或几乎白色的晶状粉末,易溶于水和乙醇,几乎不溶于乙醚。加热时 升华。
3.Identification
First identification A, D.
Second identification B, C, D.
全句可译为:鉴别
首选鉴别试验A,D。
次选鉴别试验B,C,D。
4.Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with amantadine hydrochloride CRS.Examine the substances prepared as discs.
全句可译为:使用红外吸收分光光度法检测(附录2.2.24),将所获图谱与盐酸金刚烷胺参比标准进行对比。检测时将供试品制成圆片状。
5.To 0.1 g add 1 ml of pyridine R, mix and add 0.1 ml of acetic anhydride R.
全句可译为:取供试品0.1克,加入1毫升吡啶R,摇匀,并加入0.1毫升乙酐R。
6.The precipitate, washed with water R and dried in vacuo at 60℃ for 1 h, melts (2.2.14)at 147℃ to 151℃.
全句可译为:沉淀物,经水洗,在真空中60℃干燥1小时,熔点为147℃至151℃ (附录2.2.14)。
7.1 ml of solution S (see Tests)gives reaction (a)of chlorides (2.3.1).
全句可译为:取溶液S 1毫升,(依法测定),出现氯化物反应(a)(附录2.3.1)。
8.Appearance of solution
Solution S is clear (2.2.1)and not more intensely coloured than reference solution Y7(2.2.2, Method II).
全句可译为:溶液外观
溶液S澄明,且颜色不深于对照溶液Y7(附录2.2.2,方法II)。
9.Related substances
Examine by gas chromatography (2.2.28).
全句可译为:相关物质
使用气相色谱法测定(附录2.2.28)。
10.Test solution
Dissolve 0.10 g of the substance to be examined in 2 ml of water R.Add 2 ml of a 200 g/l solution of sodium hydroxide R and 2 ml of chloroform R.Shake for 10 min.Separate the chloroform layer, dry over anhydrous sodium sulphate R and filter.
全句可译为:供试品溶液
取供试品0.10克,溶解于2毫升水R。加入2毫升浓度为200克/升的氢氧化钠R溶液和2毫升氯仿R,摇动10分钟。分离氯仿层,置于无水硫酸钠R上干燥,并过滤。
11.The chromatographic procedure may be carried out using:
— a glass column 1.8 m long and 2 mm in internal diameter with a packing prepared as follows:mix 19.5 g of silanised diatomaceous earth for gas chromatography R with 60 ml of a 3.3 g/l solution of potassium hydroxide R in methanol R and evaporate the solvent under reduced pressure while rotating the mixture slowly (support); dissolve 0.4 g of low-vapourpressure hydrocarbons (type L)R in 60 ml of toluene R (dissolution requires up to 5 h), add this solution to the support and evaporate the solvent under reduced pressure while rotating the mixture slowly.
全句可译为:色谱层析法可按下列步骤进行:
玻璃色谱柱长1.8米,内径2毫米,内装填充剂。填充剂的配制方法如下:取供气相分色谱析用硅烷化硅藻土19.5克,与60毫升浓度为3.3克/升氢氧化钾甲醇溶液混匀,在减压下蒸发溶剂,同时缓慢转动混合物(载体);取0.4克低蒸汽压烃R(L型)溶解于60毫升甲苯(溶解过程需5小时),将此溶液加入载体并在减压下将溶剂蒸发,同时缓慢转动混合物。
12.— nitrogen for chromatography R as the carrier gas at a flow rate of 30 ml/min,
— a flame-ionisation detector.
全句可译为:使用氮气作为气相色谱法用气,流速30毫升/分钟,
使用火焰电离检测器。
13.Programme the temperature of the column linearly at a rate of 6℃ per minute from 100℃ to 200℃.Maintain the temperature of the injection port at 220℃ and that of the detector at 300℃.
全句可译为:将色谱柱温度上升率设定为6℃/分钟,呈线性由100℃上升至200℃。将注入口的温度保持在220℃,检测器温度保持在300℃。
14.Continue the chromatography for a period at least 2.5 times the retention time of the principal peak.
全句可译为:色谱分析的时间至少应为主峰保留时间的2.5倍。
15.In the chromatogram the sum of the area of any peaks apart from that corresponding to amantadine does not exceed 1 percent of the total area of the peaks; no peak, apart from that corresponding to amantadine, has an area exceeding 0.3 percent of the total area.Disregard the peak corresponding to the solvent during the evaluation.
全句可译为:色谱图上,除金刚烷胺的峰面积外,其余任何峰面积不得大于总面积的1%;除金刚烷胺的峰面积外,任何峰面积不得大于总面积的0.3%。计算过程中,溶剂峰面积忽略不计。
16.Heavy metals (2.4.8)
12 ml of solution S complies with limit test A for heavy metals (20 ppm).Prepare the standard using lead standard solution (2 ppm Pb)R.
全句可译为:重金属(附录2.4.8)
取溶液S12毫升依法检查,符合重金属极限试验A(20ppm)。用铅标准溶液(2 ppm Pb)R 配制标准溶液。
17.Water (2.5.12)
Not more than 0.5 percent, determined on 2.000 g by the semi-micro determination of water.
全句可译为:水分(附录2.5.12)
不超过0.5%,取本品2 000克,用半微量测定法测定水分。
18.Carry out a potentiometric titration (2.2.20), using 0.1M sodium hydroxide.Read the volume added between the two points of inflexion.
1 ml of 0.1M sodium hydroxide is equivalent to 18.77 mg of C10H18ClN.
全句可译为:用0.1 M氢氧化钠溶液进行电位滴定(用电位计确定滴定终点)(附录2.2.20)。记录曲线上两拐点间增加值。
1毫升(0.1 M)氢氧化钠滴定液相当于18.77毫克的C10H18ClN。
Comprehension Questions
1.It is a white or almost white, crystalline powder, freely soluble in water and in alcohol, practically insoluble in ether and sublimes on being heated.
2.B.To 0.1 g add 1 ml of pyridine R, mix and add 0.1 ml of acetic anhydride R.Heat to boiling for about 10 s.Pour the hot solution into 10 ml of dilute hydrochloric acid R, cool to 5℃ and filter.The precipitate, washed with water R and dried in vacuo at 60℃ for 1 h, melts (2.2.14)at 147℃ to 151℃.
3.Dissolve 2.5 g in carbon dioxide-free water R and dilute to 25 ml with the same solvent.
4.It is clear (2.2.1)and not more intensely coloured than reference solution Y7(2.2.2, Method II).
5.Dilute 2 ml of solution S to 10 ml with carbon dioxide-free water R.Add 0.1 ml of methyl red solution R and 0.2 ml of 0.01M sodium hydroxide.The solution is yellow.Add 0.4 ml of 0.01M hydrochloric acid.The solution is red.
6.Dissolve 0.10 g of the substance to be examined in 2 ml of water R.Add 2 ml of a 200 g/l solution of sodium hydroxide R and 2 ml of chloroform R.Shake for 10 min.Separate the chloroform layer, dry over anhydrous sodium sulphate R and filter.
7.— a glass column 1.8 m long and 2 mm in internal diameter with a packing prepared as follows:mix 19.5 g of silanised diatomaceous earth for gas chromatography R with 60 ml of a 3.3 g/l solution of potassium hydroxide R in methanol R and evaporate the solvent under reduced pressure while rotating the mixture slowly (support); dissolve 0.4 g of low-vapourpressure hydrocarbons (type L)R in 60 ml of toluene R (dissolution requires up to 5 h), add this solution to the support and evaporate the solvent under reduced pressure while rotating the mixture slowly,
— nitrogen for chromatography R as the carrier gas at a flow rate of 30 ml/min,
— a flame-ionisation detector.
8.Programme the temperature of the column linearly at a rate of 6℃ per minute from 100℃ to 200℃.Maintain the temperature of the injection port at 220℃ and that of the detector at 300℃.
9.In the chromatogram the sum of the area of any peaks apart from that corresponding to amantadine does not exceed 1 percent of the total area of the peaks; no peak, apart from that corresponding to amantadine, has an area exceeding 0.3 percent of the total area.Disregard the peak corresponding to the solvent during the evaluation.
10.Dissolve 0.150 g in a mixture of 5.0 ml of 0.01M hydrochloric acid and 50 ml of alcohol R.Carry out a potentiometric titration (2.2.20), using 0.1M sodium hydroxide.Read the volume added between the two points of inflexion.
1 ml of 0.1M sodium hydroxide is equivalent to 18.77 mg of C10H18ClN.
Unit 6
Exercise
Task 1
1.Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C8H11N5O3, calculated on the anhydrous basis.
2.It should be preserved in tight containers, and stored at room temperature, protected from light and moisture.
3.The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay and limit for guanine.
4.Not exceeding 6%.
5.Prepare a filtered and degassed solution of glacial acetic acid in water (1 in 1000).Make adjustments if necessary (see System Suitability under Chromatography <621>).
6.Dissolve accurately weighed quantities of USP Acyclovir RS and guanine in 0.1 N sodium hydroxide, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having known concentrations of about 0.1 mg of each per ml.
7.Dissolve about 100 mg of Acyclovir, accurately weighed, in 20 ml of 0.1 N sodium hydroxide in a 200-ml volumetric flask, dilute with water to volume, and mix.Transfer 10.0 ml of this solution to a 50-ml volumetric flask, dilute with 0.01 N sodium hydroxide to volume, and mix.
8.The liquid chromatograph is equipped with a 254-nm detector and a ▲4.6-mm×25-cm▲USP30column that contains packing L1.The flow rate is about 3 ml per minute.
9.Inject System suitability solution 1 into the liquid chromatograph, record the peak responses as directed for Procedure:the resolution, R, between acyclovir and guanine is not less than 2.0; the tailing factor for the analyte peak is not more than 2; and the relative standard deviation for replicate injections for the acyclovir peak is not more than 2.0%.
10.The formula:1000C(rU/rS)is taken to calculate the quantity of guanine in the portion of Acyclovir.
11.In the formula, C is the concentration, in mg per ml, of USP Acyclovir RS in the Standard preparation; and rUand rSare the peak responses due to cyclovir in the Assay preparation and the Standard preparation, respectively.
Task 2
游离水杨酸限量——取供试品2.5克,溶于充足的酒精中配制成25.0毫升。分别向两根比色管内加入48毫升水和1毫升新鲜配制的稀硫酸铁氨溶液(硫酸铁氨溶液的配制方法:取1毫升1 N的盐酸,加入2毫升硫酸铁氨试液,并加水稀释至100毫升)。用移液管向其中一根比色管移入1毫升水杨酸标准水溶液,每毫升含0.10毫克水杨酸。向另一根比色管移入1毫升1∶10阿司匹林溶液。分别将两根比色管摇匀:30秒后,第二根比色管内溶液的颜色不得深于含水杨酸(0.1%)比色管。
含量测定——精密称定1.5克阿司匹林放入量瓶,加入50.0毫升0.5 N氢氧化钠滴定液,并用小火煮沸10分钟。加入酚酞试液,用0.5 N硫酸滴定液中和过量的氢氧化钠。重复进行空白试验,(见滴定法项下剩余滴定,附录541)。每毫升0.5 N氢氧化钠滴定液相当于C9H8O445.04毫克。
Notes to Supplementary Reading
1.N-[4-[[(2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-folic acid.
N-[p-[[(2-Amino-4-hydroxy-6-pteridinyl)methyl]amino]-benzoyl]-L-glutamic acid
全句可译为:本品为N-[4[[(2-氨基-1,4二氢-4-氧代-6蝶啶)甲基]氨基]-苯甲酰基]-叶酸。
也称N-[p[[(2-氨基-4-羟基-6-蝶啶)甲基]氨基]-苯甲酰基]-L-谷氨酸
2.Identification, Ultraviolet Absorption <197U>—
Solution:10 μg per ml.
Medium:Sodium hydroxide solution (1 in 250).
Ratio:A256/A365, between 2.80 and 3.00.
全句可译为:鉴别 (照)紫外吸收光谱法(附录197U)测定。
溶液:10微克/毫升。
溶媒:氢氧化钠溶液(1∶250)。
比率:A256/A365,在2.80—3.00之间。
3.Water—Proceed as directed for Method I 〈921〉, except to stir the methanol solvent prior to and during the addition of the test specimen, and during the titration:not more than 8.5% is found.
全句可译为:水分——除在添加试验样品前及过程中与滴定过程中搅动溶剂甲醇外,按附录921方法I说明进行测定,含水分不得过8.5%。
4.Chromatographic purity —
3 N phosphoric acid, 6 N ammonium hydroxide, Internal standard solution, Standard stock solution, Standard preparation, and Chromatographic system—Proceed as directed in the Assay.
全句可译为:色谱纯度
准备3N磷酸、6N氢氧化氨、内标准液、标准贮备液、标准液及色谱法系统——按测定项下的说明进行。
5.Procedure—Inject about 10 μl of the Test solution into the chromatograph, and allow the Test solution to elute for not less than 2 times the retention time of folic acid.
全句可译为:步骤——向色谱仪内注入约10微升试验溶液,试验溶液洗提的时间不少于叶酸(主峰)保留时间的2倍。
6.Record the chromatogram, and measure the responses of all the peaks.The sum of the area of all peaks, other than that due to folic acid, is not greater than 2.0%.
全句可译为:记录色谱图,测量所有峰面积。所有峰面积的总和,除叶酸峰面积外,不大于2.0%。
7.Assay— [NOTE—Use low actinic glassware throughout the following procedure.]
3 N phosphoric acid—Dissolve 9.8 g of phosphoric acid in 100 ml of water.
6 N ammonium hydroxide—Dilute 40 ml of ammonium hydroxide with water to 100.0 ml.
全句可译为:含量测定 [注意——全过程使用低色散光学玻璃器皿。]
3N磷酸(的配制)——取磷酸9.8克,溶于100毫升水中。
6N氢氧化氨(的配制)——取氢氧化氨40毫升,加水稀释至100毫升。
8.Add 15.0 ml of solution of 0.5 M tetrabutylammonium hydroxide in methanol, ...
全句可译为:加入15.0毫升0.5M四丁基氢氧化铵的甲醇溶液,……
9.Standard stock solution—Prepare a solution of USP Folic Acid RS in Mobile phase having a known concentration of about 1 mg per ml.[NOTE—Use 1 ml of 10% ammonium hydroxide to dissolve the folic acid for every 100 ml of Standard stock solution prepared.]
全句可译为:标准贮备液的配制——用流动相配制成(已知)浓度约为1毫克/毫升的《美国药典》叶酸参比标准溶液。[注意——每配制100毫升标准贮备液需使用1毫升氢氧化氨溶液溶解叶酸。]
10.Standard preparation—Transfer 4.0 ml of Standard stock solution to a 50-ml volumetric flask, add 4.0 ml of Internal standard solution, dilute with Mobile phase to volume, and mix.
全句可译为:标准溶液的配制——量取4.0毫升标准贮备液置于50毫升量瓶,加入4.0毫升内标准溶液,用流动相稀释至刻度,摇匀。
11.Chromatographic system (see Chromatography<621>)— The liquid chromatograph is equipped with a 280-nm detector and a 4.0-mm×25-cm column that contains packing L1.The flow rate is about 1.2 ml per minute.
全句可译为:色谱法系统(见色谱法,附录621)——液相色谱仪配有一个280纳米探测器和一根4.0毫米X25厘米,内装L1填充剂的色谱柱。流速约为1.2毫升/分钟。
12.Procedure—Separately inject equal volumes (about 10 μl)of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks.
全句可译为:步骤——分别向色谱仪注入等量的(约10微升)标准溶液和供试品溶液,记录色谱图,测量主峰的峰面积。
13....in which C is the concentration, in mg per ml, of USP Folic Acid RS, on the anhydrous basis, in the Standard preparation; and RUand RSare the ratios of the response of the folic acid peak to that of the methylparaben peak obtained from the Assay preparation and Standard preparation, respectively.
全句可译为:……公式中C为标准溶液中美国药典参比标准叶酸浓度,单位是毫克/毫升,按干燥品计算。RU与 RS分别是供试品溶液与标准溶液所获得的叶酸和羟苯甲酯峰面积的比率。
Comprehension Questions
1.They are C19H19N7O6and:441.40 respectively.
2.It’s N-[4-[[(2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-folic acid or N-[p-[[(2-Amino-4-hydroxy-6-pteridinyl)methyl]amino]-benzoyl]-L-glutamic acid.
3.It should be preserved in well-closed, light-resistant containers.
4.Proceed as directed for Method I<921>, except to stir the methanol solvent prior to and during the addition of the test specimen, and during the titration:not more than 8.5% is found.
5.Inject about 10 μl of the Test solution into the chromatograph, and allow the Test solution to elute for not less than 2 times the retention time of folic acid.Record the chromatogram, and measure the responses of all the peaks.The sum of the area of all peaks, other than that due to folic acid, is not greater than 2.0%.
6.Low actinic glassware should be used throughout the procedure.
7.Prepare a solution of USP Folic Acid RS in Mobile phase having a known concentration of about 1 mg per ml.[NOTE—Use 1 ml of 10% ammonium hydroxide to dissolve the folic acid for every 100 ml of Standard stock solution prepared.]
8.Transfer 4.0 ml of the Assay stock solution to a 50-ml volumetric flask, add 4.0 ml of Internal standard solution, dilute with Mobile phase to volume, and mix.
9.Separately inject equal volumes (about 10 μl)of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks.
10.The formula 1250C(RU/RS)is used to calculate the quantity of C19H19N7O6in the portion of Folic Acid.
Unit 7
Exercises
Task 1
1.TCM is based on a belief in yin and yang—defined as opposing energies
2.When there’s too little or too much qi in one of the body’s energy pathways called meridians, or when the flow of qi is blocked, illness results.
3.The ultimate goal of TCM treatment is to balance the yin and yang in our lives by promoting the natural flow of qi.
4.Herbal medicine and acupuncture, including theory, practice, diagnosis, and treatment, were recorded in classical Chinese texts and refined over many centuries.
5.In 1971, when New York Times reporter James Reston wrote the story of his having acupuncture for pain after an emergency appendix operation.
6.Practices used in TCM include acupuncture and acupressure, moxibustion (burning an herb near the skin), herbal medicine, nutrition, Chinese massage (called tui na)and exercise (such as tai chi and qi gong which combine movement with meditation).
7.In TCM, the body’s internal organs are not thought of as individual structures, but as complex networks.According to TCM, there are five organ systems (kidney, heart, spleen, liver and lung), through which qi flows via meridians.
8.Some of the conditions for which TCM is known to be particularly helpful include obesity, diabetes and its complications such as retinopathy (damage to the retina located in the back of the eye), high cholesterol, male and female fertility disorders, Alzheimer’s disease, digestive disorders (such as irritable bowel syndrome), and recurrent cystitis (inflammation of the bladder).
9.TCM may also be an effective treatment for the following ailments:allergies, sinusitis, addictions, pain (including childbirth and abdominal), menopausal symptoms, osteoporosis, arthritis, infections (respiratory, bladder, vaginal), sleep disorders, stress and constipation.
10.Already there are 35 Oriental medicine training programs in the United States.Recently, nine Chinese medical institutions and Ohio University College of Osteopathic Medicine joined forces to study how TCM can be applied to Western medicine.Similarly, the University of Pittsburgh created an International TCM Center to coordinate research efforts with TCM institutions in China.Future research studies and clinical trials on TCM are needed to find out exactly how it works, and its effectiveness, safety and cost.
Task 2
传统中医药材(TCMM)如今已经成为中医的一个新的分支。20世纪80年代,在传统天然药品的研究上取得了长足进展。从中药材中萃取青蒿素被认为是抗疟疾药的重要突破。对青蒿素的研究及用中药治疗泌尿系统感染已经获得了艾伯特·爱因斯坦世界科学奖。随着中医药材的转化,打开了一条寻找新药品和新疗法的道路。为了确保原料药的质量和供应,已经建立起了600多家生产基地和13 000多个药物种植园。目前已经有800多家中药制药厂,年产量达 400 000多吨,生产品种超过5 000种。
Notes to Supplementary Reading
1.Originating in China more than 2,000 years ago, acupuncture began to become better known in the United States in 1971, when New York Times reporter James Reston wrote about how doctors in China used needles to ease his pain after surgery.
originating in China more than 2,000 years ago是分词短语,代替状语从句,相当于:Although acupuncture was originated in China more than 2,000 years ago(尽管针灸2 000多年前起源于中国)。
acupuncture began to become better known in the United States in 1971是本句的主要部分:从1971年开始,针灸才更为美国人所了解。
when在本句中是关系副词,起从属连词作用,其先行词为1971,后面引导一个非限制性定语从句,意思是“就在那时”。
when New York Times reporter James Reston wrote about how doctors in China used needles to ease his pain after surgery:就在那一年,《纽约时报》记者James Reston报导中国医生如何用针给他止住手术后疼痛(的消息)。
2.The acupuncture technique that has been most studied scientifically involves penetrating the skin with thin, solid, metallic needles that are manipulated by the hands or by electrical stimulation.
本句:The acupuncture technique involves penetrating the skin with metallic needles是主要成分:针灸技术涉及用金属针穿透皮肤。
that has been most studied scientifically 是定语从句,修饰technique,即:已经进行过最多科学研究的针灸技术涉及用金属针穿透皮肤。
that are manipulated by the hands or by electrical stimulation是定语,修饰金属针metallic needles:用手或电流刺激对针进行操作。
3.The report from a Consensus Development Conference on Acupuncture held at the National Institutes of Health (NIH)in 1997 stated that acupuncture is being “widely” practiced—by thousands of physicians, dentists, acupuncturists, and other practitioners—for relief or prevention of pain and for various other health conditions.
本句的主要成分是:the report stated(报告宣称),that acupuncture is being “widely” practiced是stated的宾语从句,报告宣称,针灸正在得到广泛的使用。
from a Consensus Development Conference on Acupuncture held at the National Institutes of Health (NIH)in 1997是定语短语,修饰report,即:一份来自1997年在国家卫生研究院举行的一致同意发展针灸会议的报告宣称;
by thousands of physicians, dentists, acupuncturists and other practitioners是使用practice的执行者:数千名内科医生、牙科医生、针灸师及其他执业人员广泛使用。
for relief or prevention of pain and for various other health conditions;是介词短语,表示上述人员使用针灸的目的:用于缓解或预防多种疾病引起的疼痛。
4.According to the 2002 National Health Interview Survey—the largest and most comprehensive survey of complementary and alternative medicine (CAM)use by American adults to date—an estimated 8.2 million U.S.adults had ever used acupuncture, and an estimated 2.1 million U.S.adults had used acupuncture in the previous year.
全句可译为:根据2002年的全国健康访问调查——这是一次对美国成年人使用补充和替代医疗进行的最大且最全面的调查——估计有820万美国成年人曾经接受过针灸治疗,另估计有210万美国成年人在前一年接受过针灸治疗。
5.Improper needle placement, movement of the patient, or a defect in the needle can cause soreness and pain during treatment.
全句可译为:进针穴位选择不当,病人在治疗中移动,或针的缺陷能引起病人在治疗过程中的疼痛。
6.The U.S.Food and Drug Administration (FDA)approved acupuncture needles for use by licensed practitioners in 1996.The FDA requires that sterile, nontoxic needles be used and that they be labeled for single use by qualified practitioners only.
全句可译为:FDA1996年批准了供执业医生使用的针灸针,同时要求必需使用无菌、无毒的针灸针,并必须在标签上标明仅供合格执业人员一次性使用。
7.Relatively few complications from the use of acupuncture have been reported to the FDA in light of the millions of people treated each year and the number of acupuncture needles used.
in (the)light of:鉴于,由于,考虑到
in light of the millions of people treated each year and the number of acupuncture needles used:鉴于每年有数百万人接受针灸治疗及使用的针灸针的数量
relatively few complications from the use of acupuncture:因针灸引起的并发症相对地说是很少的
全句可译为:考虑到每年有数百万人接受针灸治疗及使用的针灸针的数量,FDA接到报告因针灸引起的并发症相对地说是很少的。
8.When not delivered properly, acupuncture can cause serious adverse effects, including infections and punctured organs.
全句可译为:当针灸方法不得当时,能引起包括感染和刺透器官等严重副作用。
9....but results have been mixed because of complexities with study design and size, as well as difficulties with choosing and using placebos or sham acupuncture.
全句可译为:……由于研究设计、研究规模及选择和使用安慰剂或假针灸的困难等复杂性,研究结果混淆不清(未能取得确定的研究结果)。
10.However, promising results have emerged, showing efficacy of acupuncture, for example, in adult postoperative and chemotherapy nausea and vomiting and in postoperative dental pain.
全句可译为:然而,已经出现了可喜的效果,例如在成年人手术后、化疗后恶心呕吐及牙科手术后止痛等方面,均显示了针灸的疗效。
11....in which acupuncture may be useful as an adjunct treatment or an acceptable alternative or be included in a comprehensive management program.
全句可译为:……在这些情况下,针灸可以作为有用的辅助治疗手段,或者是可以接受的选择,或者包括在综合治疗方案中。
12.An NCCAM-funded study recently showed that acupuncture provides pain relief, improves function for people with osteoarthritis of the knee, and serves as an effective complement to standard care.
全句可译为:近来国家补充和替代医学研究中心资助的一项研究表明:针灸可以缓解膝骨关节炎患者的疼痛和改善功能,并作为标准医疗方法的有效补充。
Comprehension Questions
1.Acupuncture began to become better known in the United States in 1971, (when New York Times reporter James Reston wrote about how doctors in China used needles to ease his pain after surgery).
2.The term acupuncture describes a family of procedures involving stimulation of anatomical points on the body by a variety of techniques.
3.The acupuncture technique that has been most studied scientifically involves penetrating the skin with thin, solid, metallic needles that are manipulated by the hands or by electrical stimulation.
4.An estimated 8.2 million U.S.adults had ever used acupuncture, and an estimated 2.1 million U.S.adults had used acupuncture in the previous year.
5.Acupuncture needles are metallic, solid and hair-thin.
6.People experience acupuncture differently, but most feel no or minimal pains as the needles are inserted.
7.The FDA requires that sterile, nontoxic needles be used and that they be labeled for single use by qualified practitioners only.
8.Relatively few complications from the use of acupuncture have been reported to the FDA in light of the millions of people treated each year and the number of acupuncture needles used.Still, complications have resulted from inadequate sterilization of needles and from improper delivery of treatments.
9.Practitioners should use a new set of disposable needles taken from a sealed package for each patient and should swab treatment sites with alcohol or another disinfectant before inserting needles.
10.There have been many studies on acupuncture’s potential usefulness, but results have been mixed because of complexities with study design and size, as well as difficulties with choosing and using placebos or sham acupuncture.
Unit 8
Task 1
1.Influenza vaccination programs.
2.Yes, they are.
3.HCP might include (but are not limited to)physicians, nurses, nursing assistants, therapists, technicians, emergency medical service personnel, dental personnel, pharmacists, laboratory personnel, autopsy personnel, students and trainees, contractual staff not employed by the health-care facility, and persons (e.g., clerical, dietary, house-keeping, laundry, security, maintenance, billing, and volunteers)not directly involved in patient care but potentially exposed to infectious agents that can be transmitted to and from HCP and patients.
4.63%
5.Because of increasing of immunization.
6.The misconception that the vaccine causes influenza, and the mistaken belief that they are not at risk.
7.Yes, it is possible for a healthy adult to unknowingly spread the virus to patients at high risk for serious complications from influenza.
8.Some states and health agencies have adopted mandatory immunization programs to help decrease the likelihood of contracting influenza and the chance of infecting others.
Task 2
疫苗,与所有由 FDA管制的产品一样,经过实验室和临床数据的严格审查,以确保它的安全性、有效性、纯度和效能。批准上市的疫苗可能还需要进行更多研究,以进一步评估疫苗,解决它的安全性、有效性或可能产生的副作用的相关具体问题。
根据美国疾病控制与预防中心的资料,疫苗已经将可预防的传染病降到空前低点,并且现在很少人再遭受麻疹、百日咳和其他病症的毁灭性影响。
生物评价和研究中心调控疫苗产品。这些产品中许多都是对减少可预防的疾病起重大意义的儿童疫苗。
Notes to Supplementary Reading
1.Vaccines work by triggering a response by the body’s immune system when administered.
该句中work 为不及物动词,意思为“起作用,运作”。后面的by triggering a response,by the body’s immune system是两个介词短语做宾语。
全句翻译为:疫苗注射时通过人体免疫系统起作用。
2.Vaccines stimulate the body to make antibodies—proteins that specifically recognize and target the disease-causing bacteria and viruses, and help eliminate them from the body before they cause disease.
其中that引导的是定语从句修饰前面的proteins。
全句可译为:疫苗刺激机体产生抗体,即蛋白质,该物质能专门识别,瞄准疾病导致的细菌和病毒并且帮助他们在机体产生疾病之前消灭它。
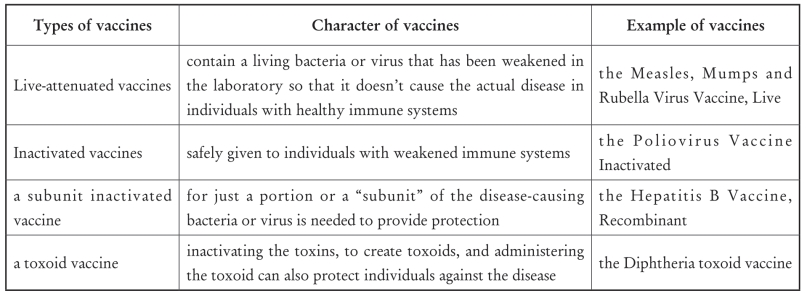
3.There are several types of vaccines:live-attenuated, inactivated (whole or subunit), and toxoids.
全句可译为:疫苗有很多种:减毒活疫苗、灭活疫苗(全病毒灭活或亚单位灭活)和类毒素。
4.Live-attenuated vaccines contain a living bacteria or virus that has been weakened in the laboratory so that it doesn’t cause the actual disease in individuals with healthy immune systems.
全句翻译为:减毒活疫苗含有在实验室里被减弱了的活菌或病毒,以便它不会引起有健康免疫系统的个体实际疾病。
5.Some bacteria cause illness by secreting a poison or toxin.
全句可译为:有些细菌通过分泌毒物或毒素产生疾病。
Comprehension Questions
Unit 9
Exercises
Task 1
1.The quality control unit shall have the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling, and drug products and the authority to review production records to assure that no errors have occurred or, if errors have occurred, that they have been fully investigated.
2.Each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience, or any combination thereof, to enable that person to perform the assigned functions.
3.Each person responsible for supervising the manufacture, processing, packing, or holding of a drug product shall have the education, training, and experience, or any combination thereof.
4.Personnel engaged in the manufacture, processing, packing, or holding of a drug product shall wear clean clothing appropriate for the duties they perform.
5.They shall have sufficient education, training, and experience, or any combination thereof, to advise on the subject for which they are retained.
Task 2
从事药品生产、加工、包装或存贮的人员,应穿着适合于其履行职责的清洁衣服。按需要,应在头部、脸部、手部、臂部穿保护外罩,防止药物受到污染。
Notes to Supplementary Reading
1.Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or labeling of a drug product.
这个句子中,upon receipt and before use in packaging or labeling of a drug product是并列状语。其中,upon receipt意思为“接收”。
全句可译为:在接收或用于药品包装和贴标签前,有代表性地对其取样与检查。
2.Any labeling or packaging materials that do not meet such specifications shall be rejected to prevent their use in operations for which they are unsuitable.
这个句子中,Any labeling or packaging materials that do not meet such specifications 是个定语从句,operations for which they are unsuitable也是个定语从句。
全句可译为:任何不符合规格标准的标签和包装材料,应被拒绝生产,以防止它们在操作中被不适当地使用。
3.Records shall be maintained for each shipment received of each different labeling and packaging material indicating receipt, examination or testing, and whether accepted or rejected.
这个句子中, received of each different labeling and packaging material做定语修饰each shipment,而indicating receipt, examination or testing, and whether accepted or rejected也是做定语,修饰records。
全句可译为:收货记录应该保留,因为每次发货的每个不同标签和包装材料都表明接收、检查或检测,以及是否接收或拒收。
4.Use of gang printing of labeling for different drug products or different strengths or net contents of the same drug product, is prohibited unless the labeling from gang-printed sheets is adequately differentiated by size, shape, or color.
这个句子中,for different drug products or different strengths or net contents of the same drug product 修饰use of gang printing of labeling, 意思为“不同药品或规格或净含量时使用联合印刷”。
全句可译为:不同药品或规格或净含量时使用联合印刷是禁止的,除非联合印刷页上的贴签得到足够的区别,比如大小、形状或颜色。
5.Dedication of labeling and packaging lines to each different strength of each different drug product.
这个句子中,dedication原意为“奉献”,在此句中意思为“专用”。 each different strength在这个句子中应译为“各个不同的剂量”。
全句可译为:每个不同剂量的不同药品应有专用贴签和包装线。
6.Printing devices on, or associated with, manufacturing lines used to imprint labeling upon the drug product unit label or case shall be monitored to assure that all imprinting conforms to the print specified in the batch production record.
这个句子中, on, or associated with, manufacturing lines修饰pringting devices,起到定语修饰作用,可译为“与产品生产线有联系的打印设备”。used to imprint labeling upon the drug product unit label or case 做定语修饰manufacturing lines。
全句译为:用于产品生产线上打印药品标签批号或与其有联系的打印设备,应监控以确保所有的打印与产品批号记录中标明的印刷品一致。
Comprehension Questions
1.Any labeling or packaging materials that do not meet such specifications shall be rejected to prevent their use in operations for which they are unsuitable.
2.Records shall be maintained for each shipment received of each different labeling and packaging material indicating receipt, examination or testing, and whether accepted or rejected.
3.Obsolete and outdated labels, labeling, and other packaging materials shall be destroyed.
4.If cut labeling is used, packaging and labeling operations shall include one of the following special control procedures:
(1)Dedication of labeling and packaging lines to each different strength of each different drug product.
(2)Use of appropriate electronic or electromechanical equipment to conduct a 100-percent examination for correct labeling during or after completion of finishing operations; or
(3)Use of visual inspection to conduct a 100-percent examination for correct labeling during or after completion of finishing operations for hand-applied labeling.Such examination shall be performed by one person and independently verified by a second person.
5.To assure that all imprinting conforms to the print specified in the batch production record.
Unit 10
Exercises
Task 1
1.B 2.B 3.C 4.A 5.C
Task 2
国家食品安全与技术中心主任,哲学博士Charles Sizer说:“这项进展的意义在于,如果没有一个在销售前对甘蓝类蔬菜进行检测的系统,就无法保证蔬菜中没有病原菌。”FDA一个以科学发展为基础的指导性文件 “工业指南——甘蓝类蔬菜生产中灌溉用废水的采样及微生物检测”在2000财政年度就可供使用,同样,还有一个较通用的关于“减少微生物对发芽种子造成食品安全危害”的指导性文件可供使用。
Notes to Supplementary Reading
1.A “1,000-sample survey” of imported produce, under which FDA investigators collected and analyzed samples of broccoli, cantaloupe, celery, cilantro, culantro, loose-leaf lettuce, parsley, scallions, strawberries and tomatoes for Salmonella and E.coli 0157:H7 (and in most cases for Shigella), was completed in FY 2000.
本句的主要成分是A “1 000-sample survey” was completed in FY 2000:在2000财政年度,完成了一项对1 000种样品的调查。
under which 引导的是一个非限制性定语从句,对该调查进行补充说明,该从句的主要成分是:FDA investigators collected and analyzed samples:FDA调查员采集并分析了样品。
for Salmonella and E.coli→ 0157:H7 (and in most cases for Shigella)是目的状语,修饰采集和分析的目的。
全句可译为:在2000财政年度完成了一项对1 000样品的调查计划,在该计划中FDA调查员采集分析了花椰菜、罗马甜瓜、芹菜、香菜、泰国香菜、散叶莴苣(生菜)、皱叶欧芹、青葱、草莓及西红柿,对其进行沙门氏杆菌、大肠杆菌0157:H7的检测,(大多数情况下还检测了志贺杆菌)。
2.“Results varied greatly depending on the commodities being tested,” Beru says.“This kind of objective information provides invaluable science-based support for how we target our surveillance of imported produce.”
Results varied greatly depending on the commodities being tested:检测结果随着被检测产品的不同变化极大。
This kind of objective information provides invaluable science-based support for how we target our surveillance of imported produce:这种客观的信息为我们如何对进口商品进行重点检测提供了极有价值的科学依据。
3.Based on violative samples, 21 firms were placed on restricted importation, called detention without physical examination (DWPE).
本句中,based on相当于due to,做“由于”解。
violative samples:违规样品,在本句中应做“不合格样品”解。
detention without physical examination (DWPE):不必检查而必定扣留
倘若入境扣留是因外国制造商的GMP问题,FDA将采取行动将该制造商置于“进口警报”状态而受到“不必检查而必定扣留” (detention without physical examination,简称DWPE)的待遇。即当某国外制造商被确定为违反GMP规定,其所有产品均不做入关检测而统统拒绝入境。在这种情况下,唯一可以使物品放行的条件就是改正所有违反GMP规定之处。
全句可译为:由于被检测出不合格样品,21家公司受到了“不必检查而必定扣留”(简称DWPE)的进口限制。
4.In FY 2000, FDA followed up on the imported survey by initiating in May a complementary 1,000-sample assignment for domestic produce.
followed up在句中应解释为“(用进一步的行动)探究”。
全句可译为:在2000财政年度,FDA通过在5月份开始的一项对国内产品的1 000个样品的补充检测,对进口商品进行了进一步的检测。
5....to conduct regulatory follow-up, including at the grower level, when positive samples occur.
regulatory:监管性的
to conduct regulatory follow-up:(为了)进行监管性的跟踪检查
including at the grower level:包括种植者层面
该短语可译为:当样品检测出现阳性时,对包括种植者层面进行监管性的跟踪检测。
6.Also in FY 2000, FDA assisted USDA’s National Agricultural Statistical Service (NASS)in conducting a fruit and vegetable agricultural practices survey that will establish a baseline of grower and packer practices.
USDA:United States Department of Agriculture美国农业部
USDA’s National Agricultural Statistical Service (NASS):美国农业部国家农业统计服务处
本句主要成分是FDA assisted UNAS’s NASS:FDA协助美国农业部国家农业统计服务处。
baseline:在句中意思为“原始资料”。
全句可译为:也是在2000财政年度,FDA协助美国农业部国家农业统计服务处进行了一项水果蔬菜生产习惯的调查,以便建立起一套种植者和包装者常规行事的原始资料。
7.Follow up surveys will provide a measure of the degree of adoption of FDA’s “Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables.”
Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables:《关于减少新鲜水果和蔬菜微生物所致食品安全风险的导则》。
全句可译为:进一步的调查将衡量对FDA《关于减少新鲜水果和蔬菜微生物所致食品安全风险的导则》的采纳程度。
Comprehension Questions
1.A “1,000-sample survey” of imported produce, under which FDA investigators collected and analyzed samples of broccoli, cantaloupe, celery, cilantro, culantro, loose-leaf lettuce, parsley, scallions, strawberries and tomatoes for Salmonella and E.coli 0157:H7 (and in most cases for Shigella), was completed in FY 2000.
2.Of 1,003 samples, 96 percent were not contaminated with any of the three pathogens.
3.Based on violative samples, 21 firms were placed on restricted importation, called detention without physical examination (DWPE).
4.The goals of the domestic project are to collect baseline information on pathogens in produce and to conduct regulatory follow-up, including at the grower level, when positive samples occur.
5.E.coli 0157:H7 and Salmonella, and in most products Shigella are to be analyzed in the project.
6.Because these products are not grown in a sterile environment, the goal is to minimize microbial contamination to the greatest extent possible.