Text USP 30 Acyclovir
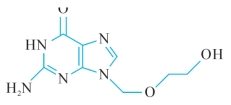
C8H11N5O3225.20
6H-Purin-6-one, 2-amino-1, 9-dihydro-9-[(2-hydroxyethoxy)methyl]-9-[(2-Hydroxyethoxy)methyl]guanine [59277-89-3].
 Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C8H11N5O3, calculated on the anhydrous basis.
Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C8H11N5O3, calculated on the anhydrous basis.
Packaging and storage—Preserve in tight containers.Store at room temperature.Protect from light and moisture.
USP Reference standards <11>— USP Acyclovir RS.
Identification—
A:Infrared Absorption <197K>.
B:The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay and limit for guanine.
Water, Method I <921>:not more than 6.0%.
Ordinary impurities <466> —
Test solution:dimethyl sulfoxide.
Standard solution:dimethyl sulfoxide.
Eluant:a mixture of chloroform, methanol, and ammonium hydroxide (80∶20∶2).
Visualization:1.
Application volume:5 μl.
Limit:1%.
Organic volatile impurities, Method V <467>:meets the requirements.
Solvent—Use dimethyl sulfoxide.
Residual solvents <467>:meets the requirements.
(Official until January 1, 2007)
Change to read:
Assay and limit for guanine —
Mobile phase—Prepare a filtered and degassed solution of glacial acetic acid in water (1 in 1000).Make adjustments if necessary (see System Suitability under Chromatography <621>).
System suitability solution 1—Dissolve accurately weighed quantities of USP Acyclovir RS and guanine in 0.1 N sodium hydroxide, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having known concentrations of about 0.1 mg of each per ml.
System suitability solution 2—Dissolve an accurately weighed quantity of guanine in 0.1 N sodium hydroxide, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having a known concentration of about 0.7 μg per ml.
Guanine standard preparation—Transfer about 8.75 mg of guanine, accurately weighed, to a 500-ml volumetric flask.Dissolve in 50 ml of 0.1 N sodium hydroxide, dilute with water to volume, and mix.Transfer 2.0 ml of this solution to a 50-ml volumetric flask, dilute with 0.01 N sodium hydroxide to volume, and mix to obtain a solution having a concentration of about 0.7 μg per ml.
Standard preparation—Dissolve about 25 mg of USP Acyclovir RS, accurately weighed, in 5 ml of 0.1 N sodium hydroxide in a 50-ml volumetric flask, dilute with water to volume, and mix.Transfer 10.0 ml of this solution to a 50-ml volumetric flask, dilute with 0.01 N sodium hydroxide to volume, and mix to obtain a solution having a known concentration of about 0.1 mg of USP Acyclovir RS per ml.
Assay preparation—Dissolve about 100 mg of Acyclovir, accurately weighed, in 20 ml of 0.1 N sodium hydroxide in a 200-ml volumetric flask, dilute with water to volume, and mix.Transfer 10.0 ml of this solution to a 50-ml volumetric flask, dilute with 0.01 N sodium hydroxide to volume, and mix.
Chromatographic system (see Chromatography <621>)— The liquid chromatograph is equipped with a 254-nm detector and a▲4.6-mm×25-cm▲USP30column that contains packing L1.The flow rate is about 3 ml per minute.Chromatograph System suitability solution 1, and record the peak responses as directed for Procedure:the resolution, R, between acyclovir and guanine is not less than 2.0; the tailing factor for the analyte peak is not more than 2; and the relative standard deviation for replicate injections for the acyclovir peak is not more than 2.0%.Chromatograph System suitability solution 2, and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—Separately inject equal volumes (about 20 μl)of the Standard preparation, the Guanine standard preparation, and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for all the peaks.Calculate the quantity, in μg, of guanine in the portion of Acyclovir taken by the formula:
1000C(rU/rS)
in which C is the concentration, in μg per ml, of guanine in the Guanine standard preparation; and rUand rSare the peak responses due to guanine in the Assay preparation and the Guanine standard preparation, respectively:not more than 0.7% of guanine is found.Calculate the quantity, in mg, of C8H11N5O3in the portion of Acyclovir taken by the formula:
1000C(rU/rS)
in which C is the concentration, in mg per ml, of USP Acyclovir RS in the Standard preparation; and rUand rSare the peak responses due to acyclovir in the Assay preparation and the Standard preparation, respectively.
注:文中计算单位因引用原文,未作规范化处理。编者
NEW WORDS AND EXPRESSIONS


NOTES
1.题录部分
Acyclovir:主标题
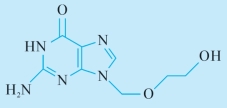
化学结构式(chemical structure)
C8H11N5O3:分子式 (chemical formula)
225.20:分子量 (molecular weight)
2.6H-Purin-6-one, 2-amino-1, 9-dihydro-9-[(2-hydroxyethoxy)methyl]-9-[(2-hydroxyethoxy)methyl]guanine:本品为6H—嘌呤—6—酮—2—氨基—1,9—二氢—9—[(2羟基乙氧基)甲基]—9—[(2—羟基乙氧基)甲基] 鸟嘌呤
3.[59277-89-3] 美国化学文摘登记号(Chemical Abstract Service Registry Number)。
4. Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C8H11N5O3, calculated on the anhydrous basis.
Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C8H11N5O3, calculated on the anhydrous basis.
符号“ ”在《美国药典》中表示正文开始。
”在《美国药典》中表示正文开始。
全句可译为:阿昔洛韦含C8H11N5O3不少于98.0%,不多于101.0%,按干品计算。
按《中国药典》表述应译为:按干燥品计算,本品含阿昔洛韦(C8H11N5O3)应为标示量的98.0%—101.0%。
5.Store at room temperature:室温保存。
药典中,对于温度的表示方式如下:
In a deep-freeze:below -15℃ 低温冷藏:低于-15℃;
In a refrigerator:2℃ to 8℃ 冷藏:2℃—8℃之间;
Cold or cool:8℃ to 15℃ 冷却:8℃—15℃之间;
Room temperature:15℃ to 25℃ 室温:15℃—25℃之间。
6.USP Reference standards <11>—USP Acyclovir RS.
《美国药典》参比标准 符合《美国药典》阿昔洛韦参比标准,附录11。
7.B:The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay and limit for guanine.
在corresponds to that中,that=the major peak主峰,以避免重复。
全句可译为:(鉴别)B:供试品溶液主峰的保留时间应与对照品溶液主峰的保留时间一致,与含量测定和鸟嘌呤限量项下获得的一致。
8.Ordinary impurities <466>—
Test solution:dimethyl sulfoxide.
Standard solution:dimethyl sulfoxide.
Eluant:a mixture of chloroform, methanol, and ammonium hydroxide (80:20:2).
Visualization:1.
Application volume:5 μl.
Limit:1%.
Visualization:1.即在254纳米处及366纳米处使用紫外光照射。Use UV light at 254 nm and at about 366 nm.附录466说明。
全句可译为:常见杂质(依法测定)附录466。
(配制)供试品溶液:二甲基亚砜
(配制)标准溶液:二甲基亚砜
(配制)洗脱液:氯仿,甲醇与氢氧化氨混合溶液(80∶20∶2)。
显影:1。
用量:5微升。
限量:1%。
9.Organic volatile impurities, Method V<467>:meets the requirements.
全句可译为:有机挥发性杂质:符合要求,附录467,方法V。
10.Residual solvents <467>:meets the requirements.
(Official until January 1, 2007)
全句可译为:溶剂残留:符合要求,附录467。
(2007年1月1日起正式标准)
11.Change to read:
Assay and limit for guanine —
Mobile phase—Prepare a filtered and degassed solution of glacial acetic acid in water (1 in 1000).Make adjustments if necessary (see System Suitability under Chromatography <621>).
全句可译为:(将测定结果)转换成读数:
含量测定和鸟嘌呤限量——
流动相——用过滤后脱气冰醋水溶液(1∶1 000)。必要时可调整(见色谱法项下的系统适应性试验,附录621)。
12.System suitability solution 1— Dissolve accurately weighed quantities of USP Acyclovir RS and guanine in 0.1 N sodium hydroxide, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having known concentrations of about 0.1 mg of each per ml.
全句可译为:系统适应性试验溶液1——精密称定一定量的《美国药典》阿昔洛韦参比标准(标准品)与鸟嘌呤,溶解于0.1 N氢氧化钠溶液,用水定量稀释,必要时可以逐步稀释,配制成已知浓度为0.1 mg/ml的溶液。
13.Guanine standard preparation—Transfer about 8.75 mg of guanine, accurately weighed, to a 500-ml volumetric flask.Dissolve in 50 ml of 0.1 N sodium hydroxide, dilute with water to volume, and mix.
全句可译为:鸟嘌呤标准溶液——精密称定约8.75毫克鸟嘌呤,放入500毫升量瓶。
溶解于50毫升0.1 N氢氧化钠溶液,用水稀释至刻度,摇匀。
14.Chromatographic system (see Chromatography <621>)— The liquid chromatograph is equipped with a 254-nm detector and a ▲4.6-mm×25-cm▲USP30column that contains packing L1.The flow rate is about 3 ml per minute.
全句可译为:色谱分析法系统(见色谱分析法,附录621)液相色谱仪配有一个254纳米检测器,一根4.6-mm×25-cm(《美国药典(第30版)》规定的)色谱柱,内装L1填充剂。流速约为3毫升/分钟。
15.Chromatograph System suitability solution 1, and record the peak responses as directed for Procedure:the resolution, R, between acyclovir and guanine is not less than 2.0; the tailing factor for the analyte peak is not more than 2; and the relative standard deviation for replicate injections for the acyclovir peak is not more than 2.0%.
全句可译为:使用色谱法分析系统适应性试验溶液1,按步骤项下的说明记录峰面积:阿昔洛韦与鸟嘌呤的分辨率R值相差不小于2.0;拖尾因子分析物峰面积不大于2。阿昔洛韦峰面积的重复注入偏差不大于2.0%。
16.Procedure—Separately inject equal volumes (about 20 μl)of the Standard preparation, the Guanine standard preparation, and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for all the peaks.Calculate the quantity, in μg, of guanine in the portion of Acyclovir taken by the formula:
全句可译为:步骤——分别向色谱仪注入等量的(约20微升)标准品溶液,鸟嘌呤标准溶液和测定(供试品)溶液,记录色谱图,测量所有的峰面积。用下列公式计算阿昔洛韦中鸟嘌呤含量,单位微克。
17....rUand rSare the peak responses due to guanine in the Assay preparation and the Guanine standard preparation, respectively:not more than 0.7% of guanine is found.Calculate the quantity, in mg, of C8H11N5O3in the portion of Acyclovir taken by the formula:
全句可译为:……rU与rS分别为鸟嘌呤供试品溶液和标准品溶液峰面积,鸟嘌呤含量不大于0.7%。用以下公式计算阿昔洛韦中C8H11N5O3的含量。
Exercises
Task 1 Answer the following questions.
1.How much C8H11N5O3should Acyclovir contain on the anhydrous basis?
2.How should Acyclovir be packed and stored?
3.What is the retention time of major peak required in Identification B?
4.What is the maximum content of water in Acyclovir?
5.How to prepare Mobile phase in Assay?
6.In which way System suitability solution 1 should be prepared?
7.How to prepare a solution for Assay?
8.What is required in Chromatographic system?
9.How to chromatograph System suitability solution 1?
10.Which formula is taken calculating the quantity, in μg, of guanine in the portion of Acyclovir?
11.What does each letter stand for in the formula taken when calculating the quantity, in mg, of C8H11N5O3in the portion of Acyclovir?
Task 2 Translate the following passage into Chinese.
Limit of free salicylic acid1— Dissolve 2.5 g in sufficient alcohol to make 25.0 ml.To each of two matched color-comparison tubes add 48 ml of water and 1 ml of a freshly prepared, diluted ferric ammonium sulfate solution2(prepared by adding 1 ml of 1 N hydrochloric acid to 2 ml of ferric ammonium sulfate TS and diluting with water to 100 ml).Into one tube pipet 1 ml of a standard solution of salicylic acid in water, containing 0.10 mg of salicylic acid per ml.Into the second tube pipet 1 ml of the 1 in 10 solution of Aspirin3.Mix the contents of each tube:after 30 seconds, the color in the second tube is not more intense than that in the tube containing the salicylic acid (0.1%).
Assay—Place about 1.5 g of Aspirin, accurately weighed, in a flask, add 50.0 ml of 0.5 N sodium hydroxide VS4, and boil the mixture gently for 10 minutes.Add phenolphthalein TS5, and titrate the excess sodium hydroxide6with 0.5 N sulfuric acid VS.Perform a blank determination (see Residual Titrations under Titrimetry7<541>).Each ml of 0.5 N sodium hydroxide is equivalent to 45.04 mg of C9H8O4.
Notes:1.free salicylic acid 游离水杨酸
2.ferric ammonium sulfate solution 硫酸铁氨溶液
3.Aspirin 阿斯匹林 4.sodium hydroxide VS 氢氧化钠滴定液
5.phenolphthalein TS 酚酞试液 6.excess sodium hydroxide 过量的氢氧化钠
7.titrimetry 滴定分析法
英、美药典的阅读与翻译(二)
三、各论文字特点 (Writing Characteristics of Monographs)
药典作为专业性很强的法典,目的在于做出种种规定,要人们严格遵守,故用词正规且严谨,经多年的修订和筛选,各款文字均有固定的词汇和习惯用法,已经高度规范化、程式化、定型化了。其特点可概括为:严谨、简练、正规、不用俗语、俚语及普通惯用法。简单归纳如下:
(一) 全篇都用一般现在时态
Definition
Amantadine hydrochloride contains not less than 98.5 per cent and not more than the equivalent of 101.0 per cent of tricyclo[3.3.1.13,7]decan-1-amine, calculated with reference to the anhydrous substance.(BP)
定义:按干燥品计算,本品盐酸金刚烷胺含三环[3.3.1.13,7] 癸烷—1—氨为标示量的98.5%—101.0%之间。
Benzylpenicillin is either the potassium salt or the sodium salt.(BP)
苄基青霉素既可是钾盐,也可是钠盐。
It complies with the requirements of the European Pharmacopoeia.
本品符合《欧洲药典》的要求。
Aspirin contains not less than 99.5% and not more than 100.5% of C9H8O4,calculated on the dried basis.(USP)
按干燥品计算,阿司匹林制品应含有不低于99.5%、不高于100.5%的C9H8O4。
It meets the requirements under Identification—Organic Nitrogenous Base.(USP)
本品符合《美国药典》鉴别项下关于有机氮碱的检测要求。
(二) 祈使句多
Related substances
Examine by gas chromatography (2.2.28).
相关物质
用气相色谱法测定(附录2.2.28)。
Preserve in tight containers. (USP)
保存于密闭容器中。
Preserve hydrous Caffeine in tight containers.(BP)
含水咖啡因应贮于密闭容器中。
Dissolve 1 g of Atropine Sulfate, accurately weighed, in 50 ml of glacial acetic acid, and titrate with 0.1 N perchloric acid VS, determining the end-point potentiometrically.(USP)
准确称取硫酸阿托品1 g,加冰醋酸50 ml使之溶解,用高氯酸滴定液(0.1 N)滴定,用电位计测定其终点。
Programme the temperature of the column linearly at a rate of 6℃ per minute from 100℃ to 200℃.Maintain the temperature of the injection port at 220℃ and that of the detector at 300℃.(BP)
将色谱柱温度上升率设定为6℃/分钟,呈线性由100℃ 上升至 200℃。将注入口的温度保持在220℃,探头的温度保持在300℃。
(三) 省略句多
1.省略主语
A white or almost white, crystalline powder, freely soluble in water and in alcohol, practically insoluble in ether.It sublimes on being heated.(BP)
性状:本品为白色或几乎白色的晶状粉末,易溶于水和乙醇,几乎不溶于乙醚。加热时升华。(省略 It is)
Very soluble in water;insoluble in fixed oils and in liquid paraffin.(BP)
本品在水中极易溶解,在固定油或液体石蜡中不溶解。(省略It is)
Silky, white crystals or a white, crystalline powder.(BP)
本品为有丝光的白色晶体或白色结晶粉末。(省略It is)
2.省略各篇主题词
To 0.1 g add 1 ml of pyridine R, mix and add 0.1 ml of acetic anhydride R.(BP)
取供试品0.1克,加入1毫升吡啶R,摇匀,并加入0.1毫升乙酐。
To 5 ml of the solution (of Atropine Sulfate)add a few drops of platinic chloride TS:No precipitate is formed.(USP)
取5 ml硫酸阿托品溶液,加入数滴氯化铂试液,不产生沉淀。
The label on the container states whether the contents are the sodium or the potassium salt.(BP)
容器的标签说明内装的是本品的钠盐或是钾盐。
3.省略实验方法:
A solution (1 in 20)responds to the tests for Sulfate<191>.(USP)
1∶20的水溶液符合硫酸盐的鉴别试验,附录191。
Residue on ignition (2815:not more than 0.05%.(USP)
炽灼残渣:不得超过0.05%附录281。
Solution S is clear (2.2.1)and not more intensely coloured than reference solution Y7(2.2.2, Method II).
溶液外观
溶液S澄明,且颜色不深于参比溶液Y7,(附录2.2.2,方法II)。(BP)
(四) 分词多
Melting temperature,Class Ia <741>:not lower than 187℃,determined after drying at 120℃ for 4 hours.(USP)
熔解温度(亦称熔点),一级A等品,取本品,在120℃干燥4小时,立即依法测定(附录741),熔解温度不得低于187℃。
When dried at 100 to 105℃ for one hour,loses not more than 0.5%of its weight.(BP)
本品在100℃—105℃干燥1小时,失重不得超过0.5%。
(五)缩写词多
1.化学元素和化学符号 (Symbols of elements and compounds)(略)
2.专业术语 (Scientific terms),如:
VS=volumetric solution 滴定液
RS=reference standard 参比标准
w/v=weight in volume重量/容积百分比
TS=test solution试(验溶)剂
(六) 用词简练
Heat to boiling 1.0 g in 50 ml of water and cool (Solution A)(BP)
取本品1.0 g,溶于50 ml水中,加热至沸腾,然后置冷(溶液A)。
Boil 1.5 g with 75 ml of water for 5 minutes,cool,add sufficient water to restore the original volume,and filter.(USP)
取本品1.5 g,加水75 ml,煮沸5分钟,置冷后加入足量的水使其恢复原有体积,并过滤。
(七) 被动语态和长句多
详见课文及注解。
四、英、美药典(各论)的不同点 (Differences between BP and USP)
英、美药典(各论)的不同点多在词汇和表达方法方面。
(一) 有的单词拼写不同
药典Pharmacopoeia (BP), Pharmacopeia (USP)
颜色colour (BP)color (USP)
气味odour (BP)odor (USP)
(二) 个别缩略语意义不同
RS:Regular Solution (BP)标准液
RS:Reference Standard (USP)参比标准
(三) 有机功能基写法不同
BP中有机功能基用英文名称的缩写表示。例如:甲基methyl:Me;乙酰基acetyl:Ac。
USP中的有机功能基则用化学分子式表示。例如:甲基:CH3—;乙酰基:CH3CO—。
(四) 性状篇小标题不同
BP用小标题Definition引导正文,USP则用符号“ ”引导,不用标题。
”引导,不用标题。
(五) 各论不同
BP和USP的重点都在检验项目,但BP稍涉及医疗用途,有Preparation制剂,Action and Use 作用和用途,Usual Dose Range常用剂量范围等项目。而USP各论中,医疗用途项目全部省略而收载于《国家处方集》(The National Formulary)之中。
(六) 化学文摘登记号不同
USP用方括号,如:[50—78—2] 表示,BP则不用方括号。
(七) 附录不同
BP称附录为Appendix,USP称为General Chapters。引用Appendix时,BP使用圆括号,括号内表明Appendix或Method的编号。而USP则将附录项目统一编号,置于“< >” 号中。
(八) 标题不同
BP各论小标题后既可是句子,也可是短语,第一个字母都用大写,与小标题间无标点相隔。USP小标题后用“—”号引导句子,用“:”引导词组。
五、翻译注意事项(Precautions)
英、美、中药典有许多相同之处,翻译时应注意各国药典的标准用名。如溶解度,三国标准相同,翻译时注意使用相应名称。

 Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C
Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C



 Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C
Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C ”在《美国药典》中表示正文开始。
”在《美国药典》中表示正文开始。 ”引导,不用标题。
”引导,不用标题。