Text BP 2007 Cefalexin Monohydrate(Ph Eur monograph 0708)
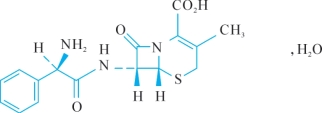
C16H17N3O4S,H2O 365.4 Preparations
23325-78-2
Action and use
Antibacterial.
Cefalexin Oral Suspension
Cefalexin Tablets
Cefalexin Capsules
Ph Eur_________________________________________________________________________
DEFINITION
(6R,7R)-7-[[(2R)-2-Amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrate.
Content
95.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Sparingly soluble in water, practically insoluble in alcohol.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison
Cefalexin monohydrate CRS.
TESTS
pH (2.2.3)
4.0 to 5.5.
Dissolve 50 mg in carbon dioxide-free water R and dilute to 10 ml with the same solvent.
Specific optical rotation (2.2.7)
+ 149 to + 158 (anhydrous substance).
Dissolve 0.125 g in phthalate buffer solution pH 4.4 R and dilute to 25.0 ml with the same solvent.
Absorbance (2.2.25)
Dissolve 50 mg in water R and dilute to 100.0 ml with the same solvent.The absorbance of the solution determined at 330 nm is not greater than 0.05.Dilute 2.0 ml of the solution to 50.0 ml with water R.Examined between 220 nm and 300 nm, the diluted solution shows an absorption maximum at 262 nm.The specific absorbance at this maximum is 220 to 245, calculated with reference to the anhydrous substance.
Related substances.
Liquid chromatography (2.2.29).
Test solution Dissolve 50.0 mg of the substance to be examined in mobile phase A and dilute to 50.0 ml with mobile phase A.
Reference solution (a)Dissolve 10.0 mg of D-phenylglycine R in mobile phase A and dilute to 10.0 ml with mobile phase A.
Reference solution (b)Dissolve 10.0 mg of 7-aminodesacetoxycephalosporanic acid CRS in phosphate buffer solution pH 7.0 R5 and dilute to 10.0 ml with mobile phase A.
Reference solution (c)Dilute 1.0 ml of reference solution (a)and 1.0 ml of reference solution (b)to 100.0 ml with mobile phase A.
Reference solution (d)Dissolve 10 mg of dimethylformamide R and 10 mg of dimethylacetamide R in mobile phase A and dilute to 10.0 ml with mobile phase A.Dilute 1.0 ml to 100.0 ml with mobile phase A.
Reference solution (e)Dilute 1.0 ml of reference solution (c)to 20.0 ml with mobile phase A.
Reference solution (f)Dissolve 10 mg of cefotaxime sodium CRS in mobile phase A and dilute to 10.0 ml with mobile phase A.To 1.0 ml of the solution add 1.0 ml of the test solution and dilute to 100 ml with mobile phase A.
Column:
— size:l=0.10 m, φ=4.6 mm,
— stationary phase:spherical octadecylsilyl silica gel for chromatography R (5 mm).
Mobile phase:
— mobile phase A:phosphate buffer solution pH 5.0 R,
— mobile phase B:methanol R2,
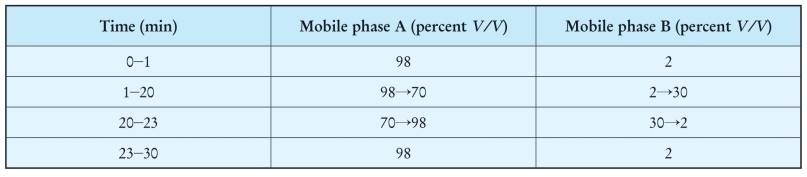
Flow rate 1.5 ml/min.
Detection Spectrophotometer at 220 nm.
Injection 20 μl; inject the test solution and reference solutions (c), (d), (e)and (f).
System suitability:
— resolution:minimum of 2.0 between the peaks due to impurity A and to impurity B in the chromatogram obtained with reference solution (c)and minimum of 1.5 between the peaks due to cefalexin and to cefotaxime in the chromatogram obtained with reference solution (f).
Limits:
— impurity B:not more than the area of the second peak in the chromatogram obtained with reference solution (c)(1.0 percent),
— any other impurity (disregard the peaks due to dimethylformamide and dimethylacetamide):not more than the area of the first peak in the chromatogram obtained with reference solution (c)(1.0 percent),
— total:not more than 3 times the area of the first peak in the chromatogram obtained with reference solution (c)(3.0 percent),
— disregard limit:the area of the second peak in the chromatogram obtained with reference solution (e)(0.05 percent).
N, N-Dimethylaniline (2.4.26, Method B)
Maximum 20 ppm.
Water (2.5.12)
4.0 per cent to 8.0 per cent, determined on 0.300 g.
Sulphated ash (2.4.14)
Maximum 0.2 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29).
Test solution Dissolve 50.0 mg of the substance to be examined in water R and dilute to 100.0 ml with the same solvent.
Reference solution (a)Dissolve 50.0 mg of cefalexin monohydrate CRS in water R and dilute to 100.0 ml with the same solvent.
Reference solution (b)Dissolve 10 mg of cefradine CRS in 20 ml of reference solution (a)and dilute to 100 ml with water R.
Column:
— size:l=0.25 m, φ=4.6 mm,
— stationary phase:octadecylsilyl silica gel for chromatography R (5 mm).
Mobile phase Methanol R, acetonitrile R, a 13.6 g/l solution of potassium dihydrogen phosphate R, water R (2:5:10:83 V/V/V/V ).
Flow rate 1.5 ml/min.
Detection Spectrophotometer at 254 nm.
Injection
20 μl.
System suitability Reference solution (b):
— resolution:minimum 4.0 between the peaks due to cefalexin and to cefradine.
Calculate the percentage content of cefalexin monohydrate.
STORAGE
Protected from light.
IMPURITIES
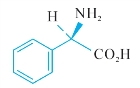
A.(2R)-2-amino-2-phenylacetic acid (D-phenylglycine),
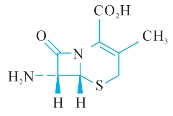
B.(6R,7R)-7-amino-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-aminodesacetoxycephalosporanic acid, 7-ADCA),
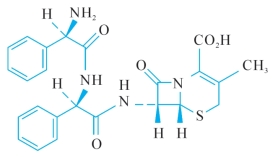
C.(6R,7R)-7-[[(2R)-2-[[(2R)-2-amino-2-phenylacetyl]amino]-2-phenylacetyl] amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,
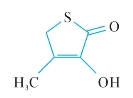
D.3-hydroxy-4-methylthiophen-2(5H)-one,
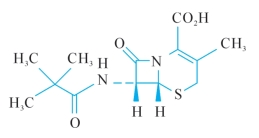
E.(6R,7R)-7-[(2,2-dimethylpropanoyl)amino]-3-methyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene-2-carboxylic acid (7-ADCA pivalamide),
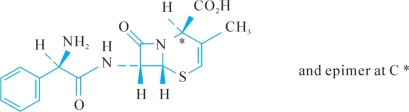
F.(2RS,6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-3-ene-2-carboxylic acid (delta-2-cefalexin).

注:文中计算单位因引用原文,未作规范化处理。编者
NEW WORDS AND EXPRESSIONS

NOTES
1.题录部分:Cefalexin Monohydrate是主标题;
 为欧盟标志,在此处表明:本各论再现《欧洲药典》。
为欧盟标志,在此处表明:本各论再现《欧洲药典》。
主标题下方斜体字(Ph Eur monograph 0708)为《欧洲药典》各论编号。
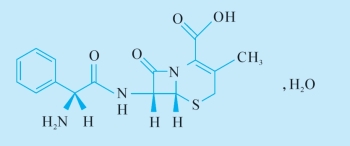
化学结构式(chemical structure)。
C16H17N3O4S,H2O:分子式 (chemical formula)
365.4:分子量 (molecular weight)
23325-78-2:《美国化学文摘》登记号 (The Chemical Abstract Service Registry Number)。
Ph Eur—— ——Ph Eur:表示引用《欧洲药典》各论部分起始和结束。
2.DEFINITION:定义,在 《英国药典》中表示正文开始。
3.(6R,7R)-7-[[(2R)-2-Amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1- azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrate.
全句可译为:本品为(6R,7R)—7[[(2R)—2氨基—2苯乙酰基]氨基]—3甲基—8氧代—5硫杂—1—氮杂双环[4.2.0]辛—2烯—2甲酸一水化合物。
4.anhydrous substance:无水物。
其意思相当于calculated with reference to anhydrous substance或calculated on dried basis,根据《中国药典》的表述,应为:按无水物(干燥品)计算。
5.IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
《英国药典》将附录统一编号,放在圆括号内,《美国药典》附录统一编号后放在尖括号内。
全句可译为:鉴别:(使用)红外吸收光度计,(依法测定,)(附录2.2.24)。
6.Solubility
Sparingly soluble in water, practically insoluble in alcohol.
全句可译为:溶解度:略溶于水,几乎不溶于乙醇。
药品的溶解度在药典中表达如下:
极易溶解的 very soluble 系指溶质1 g(ml)能在溶剂不到1 ml中溶解;
易溶的 freely soluble 系指溶质1 g(ml)能在溶剂1—不到10 ml中溶解;
可溶解的 soluble 系指溶质1 g(ml)能在溶剂10—不到30 ml中溶解;
略溶的 sparingly soluble 系指溶质1 g(ml)能在溶剂30—不到100 ml中溶解;
微溶的 slightly soluble 系指溶质1 g(ml)能在溶剂100—不到1 000 ml中溶解;
极微溶的 very slightly soluble 系指溶质1 g(ml)能在溶剂1 000—不到10 000 ml中溶解;
几乎不溶或不溶 practically insoluble 系指溶质1 g(ml)在溶剂10 000 ml中不能完全溶解。
7.pH (2.2.3)
pH值(酸度)依法测定(附录2.2.3)
Dissolve 50 mg in carbon dioxide-free water R and dilute to 10 ml with the same solvent.
carbon dioxide-free water R: 去二氧化碳水R;无二氧化碳水R
《英国药典》中,R 表示:Substance or Solution defined under Reagents,试剂项下规定的物质或溶液。
全句可译为:取本品50毫克,溶解于去二氧化碳水R,并用同种溶液(即:去二氧化碳水R)稀释至10毫升。
8.Specific optical rotation (2.2.7)
+ 149 to + 158 (anhydrous substance).
全句可译为:比旋(光)度,(附录2.2.7)。按无水物计算,+149°至+158°。
9.Dissolve 0.125 g in phthalate buffer solution pH 4.4 R and dilute to 25.0 ml with the same solvent.
全句可译为:取本品0.125克,溶解于pH值4.4的邻苯二甲酸盐缓冲溶液R中,并用同种溶液(即pH值4.4的邻苯二甲酸盐缓冲溶液R)将其稀释至25.0毫升。
10.The absorbance of the solution determined at 330 nm is not greater than 0.05.
全句可译为:在330纳米处测量溶液的吸光度,不大于0.05。
11.The specific absorbance at this maximum is 220 to 245, calculated with reference to the anhydrous substance.
the specific absorbance:吸光系数。
全句可译为:按无水物计算,在最大吸光处(330纳米处)的吸收系数为220至245之间。
12.Related substances.
Liquid chromatography (2.2.29).
全句可译为:有关物质
液相色谱法,依法测定,(附录2.2.29)。
13.Test solution
Dissolve 50.0 mg of the substance to be examined in mobile phase A and dilute to 50.0 ml with mobile phase A.
the substance to be examined=the substance being examined 供试品
全句可译为:供试品溶液(的配置)
取本品50.0毫克,溶解于流动相A,并用流动相A稀释至50.0毫升。
14.Reference solution (b)
Dissolve 10.0 mg of 7-aminodesacetoxycephalosporanic acid CRS in phosphate buffer solution pH 7.0 R5 and dilute to 10.0 ml with mobile phase A.
全句可译为:对照溶液B(的配制)
取7—氨基去乙酰氧基头孢烷酸化学参比物质10.0毫克,溶解于pH 7.0的磷酸盐缓冲剂R5,用流动相A稀释至10.0毫升。
15.Reference solution (d)
Dissolve 10 mg of dimethylformamide R and 10 mg of dimethylacetamide R in mobile phase A and dilute to 10.0 ml with mobile phase A.Dilute 1.0 ml to 100.0 ml with mobile phase A.
全句可译为:对照溶液(d)(的配制)
分别称取二甲基甲酰胺R和二甲基乙醇胺R各10毫克,溶解于流动相A,并用流动相稀释至10.0毫升。取(其中)1毫升,用流动相A稀释至100.0毫升。
16.Reference solution (f)
Dissolve 10 mg of cefotaxime sodium CRS in mobile phase A and dilute to 10.0 ml with mobile phase A.To 1.0 ml of the solution add 1.0 ml of the test solution and dilute to 100 ml with mobile phase A.
全句可译为:对照溶液(f)(的配制)
取头孢噻肟钠化学参比物质10毫克,溶解于流动相A,并用流动相A稀释至10.0毫升。取该溶液1.0毫升,加入1.0毫升供试品验溶液,并用流动相A稀释至100毫升。
17.Column:
size:l=0.10 m, φ=4.6 mm,
l=length长度;φ /faɪ/=diameter 直径
全句可译为:色谱柱:
规格:长度0.10米,直径4.6毫米。
18.Stationary phase:spherical octadecylsilyl silica gel for chromatography R (5 mm).
全句可译为:固定相:使用球形十八烷基甲硅烷基硅胶R(5毫米)进行色谱分析法。
19.Detection
Spectrophotometer at 220 nm.
全句可译为:检测
(使用)分光光度计,在220纳米处检测。
20.System suitability:
— resolution:minimum of 2.0 between the peaks due to impurity A and to impurity B in the chromatogram obtained with reference solution (c)and minimum of 1.5 between the peaks due to cefalexin and to cefotaxime in the chromatogram obtained with reference solution (f).
全句可译为:系统适应性
分辨率:在由对照溶液(c)获得的色谱图上,杂质A和杂质B双峰间最小值为2.0,在由对照溶液(f)获得的色谱图上,头孢氨苄和头孢噻肟双峰间最小值为1.5。
21.Limits:
— impurity B:not more than the area of the second peak in the chromatogram obtained with reference solution (c)(1.0 percent),
— any other impurity (disregard the peaks due to dimethylformamide and dimethylacetamide):not more than the area of the first peak in the chromatogram obtained with reference solution (c)(1.0 percent),
全句可译为:限量分析
杂质B:不大于由对照溶液(c)所获得的色谱图上第二峰面积(1.0%)。
任何其他杂质(忽略由二甲基甲酰胺和二甲基乙醇胺产生的峰值):不大于对照溶液(c)在色谱图上的第一峰面积,(1.0%)。
22.— disregard limit:the area of the second peak in the chromatogram obtained with reference solution (e)(0.05 per cent).
全句可译为:忽略不计极限:由对照溶液(e)获得的色谱图上第二峰面积(0.05%)。
23.N,N-Dimethylaniline (2.4.26, Method B)
全句可译为:N,N—二甲基苯胺(限量),依法测定(附录2.4.26,方法B)。
24.ASSAY
Liquid chromatography (2.2.29).
全句可译为:(含量)测定
使用液相色谱法(附录2.2.29)。
25.Mobile phase
Methanol R, acetonitrile R, a 13.6 g/l solution of potassium dihydrogen phosphate R, water R (2:5:10:83 V/V/V/V).
V=volume 体积。
全句可译为:流动相(配制)
甲醇R,乙腈R,磷酸二氢钾R3.6克/升, 水R按(2∶5∶10∶83 V/V/V/V)比例配制。
26.— resolution:minimum 4.0 between the peaks due to cefalexin and to cefradine.
Calculate the percentage content of cefalexin monohydrate.
全句可译为:分辨率:头孢氨苄与头孢拉定双锋间最小值为4.0。计算头孢氨苄一水化物的含量。
27.IMPURITIES
A.(2R)-2-amino-2-phenylacetic acid (D-phenylglycine),
B.(6R,7R)-7-amino-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-aminodesacetoxycephalosporanic acid, 7-ADCA),
C.(6R,7R)-7-[[(2R)-2-[[(2R)-2-amino-2-phenylacetyl]amino]-2-phenylacetyl] amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,
D.3-hydroxy-4-methylthiophen-2(5H)-one,
E.(6R,7R)-7-[(2,2-dimethylpropanoyl)amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-ADCA pivalamide),
F.(2RS,6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-3-ene-2-carboxylic acid (delta-2-cefalexin).
全句可译为:杂质(有)
A.(2R)—2—氨基—2—二苯基乙酸(D—苯基甘氨酸),
B.(6R,7R)7—氨基—3—甲基—8—氧代—5—硫杂—1—氮杂双环[4.2.0]辛—2烯—2甲酸—(7—氨基去乙酰氧基头孢烷酸),
C.(6R,7R)—7—[[(2R)—2—[[(2R)—2—氨基—2—苯乙酰基]氨基]—2—苯乙酰基]氨基]—3甲基—8—氧代—5—硫杂—1—氮杂双环[4.2.0]辛—2烯—2甲酸,
D.3—羟基—4—甲硫基苯基—2(5H)—酮,
E.(6R,7R)—7—[(2,2—二甲基丙酰基)氨基] —3—甲基—8—氧代—5—硫杂—1—氮杂双环[4.2.0]辛—2烯—2甲酸(7—ADCA新戊酰胺),
F.(2RS,6R,7R)—7—[[(2R)—2—氨基—2苯乙酰基]氨基—3甲基—8—氧代—5—硫杂—1—氮杂双环[4.2.0]辛—3—烯—2甲酸(delta—2—头孢氨苄)。
Exercises
Task 1 Answer the following questions.
1.What is the appearance of Cefalexin Monohydrate?
2.What’s the solubility of it?
3.How to prepare the solution for determining pH and its acidity?
4.How to measure the absorbance according to the monograph?
5.How to prepare the test solution for liquid chromatography when testing related substances?
6.How to prepare Reference Solution (d)?
7.Describe the column and the packing for chromatography.
8.What’s the result of resolution in system suitability?
9.How to prepare each of the solution for liquid chromatography?
10.Describe de column, stationary phase and mobile phase in Assay.
Task 2 Translate the following passage into Chinese.
Fluoroquinolonic1acid
Examine by thin-layer chromatography2(2.2.27), using as the coating substance3a suitable silica gel with a fluorescent indicator4having an optimal intensity5at 254 nm.
Test solution
Dissolve 50 mg of the substance to be examined in dilute ammonia R1 and dilute to 5 ml with the same solvent.
Reference solution
Dissolve 10 mg of fluoroquinolonic acid CRS in a mixture of 0.1 ml of dilute ammonia R1 and 90 ml of water R and dilute to 100 ml with water R.Dilute 2 ml of this solution to 10 ml with water R.
Apply to the plate65 ml of each solution.At the bottom of a chromatographic tank7, place an evaporating dish containing 50 ml of concentrated ammonia R.Close the tank and expose the plate to the ammonia vapour for 15 min.Withdraw the plate8and transfer to a chromatographic tank and develop over a path of 15 cm using a mixture of 10 volumes of acetonitrile R, 20 volumes of concentrated ammonia R, 40 volumes of methanol R and 40 volumes of methylene chloride9R.Allow the plate to dry in air and examine in ultraviolet light10at 254 nm.Any spot corresponding to fluoroquinolonic acid in the chromatogram obtained with the test solution is not more intense than the spot in the chromatogram obtained with the reference solution (0.2 percent).
Notes:1.fluoroquinolonic 氟喹啉酸 2.thin-layer chromatography 薄层色谱法
3.coating substance 涂层物(质) 4.fluorescent indicator 荧光指示器
5.optimal intensity 最佳光密度 6.plate (色谱)薄层板
7.chromatographic tank 色谱层析槽 8.withdraw the plate 取出薄层板
9.methylene chloride 二氯甲烷 10.ultraviolet light 紫外光
英、美药典的阅读与翻译(一)
一、概述
药典是由国家组织编撰并颁布的,记载药品质量标准的典籍,具有国家法规的性质。与药品说明书不同的是:药品说明书是由药品生产厂家提供的说明药品用途的医疗参考文件,供医生处方和患者参考使用。药典则是国家有关质量标准及鉴定方法的法律典籍,起着保证药品质量,保障人民用药安全的法律作用。凡不符合药典之规定的药品,均为假药。
由于英、美是世界药品的生产大国。世界许多国家和地区承认或沿用英、美药典标准。所以我们在药品的进出口中,必须经常查阅英、美药典。这两部药典也成了我们科研生产中常用的参考书。
《英国药典》是英国药品委员会(British Pharmacopoeia Commission)的正式出版物,是英国制药标准的重要来源。《英国药典》不仅为读者提供了药用和成药配方标准以及公式配药标准。(自2006年开始,《英国药典》每年修订一次。随着欧洲一体化和欧盟的成立,《英国药典》也向读者展示了许多明确分类并可参照的欧洲药典各论,英国皇家出版局(The Stationery Office)在2001年5月出版发行的《英国药典(2001)》(British Pharmacopoeia 2001)版中,收录有2 760篇医药各论,其中1 305篇出自英国本土;其余1 455篇套录自《欧洲药典》(European Pharmacopoeia)(第三版)。
在《英国药典》中,凡是套用《欧洲药典》的各论,均在主标题的右侧标有欧盟徽章(12颗五角星组成的圆圈)(Monographs of the European Pharmacopoeia are distinguished by a chaplet of stars against the title and by reference to the European Pharmacopoeia monograph number included immediately below the title in italics.The beginning and end of text from the European Pharmacopoeia are denoted by means of horizontal lines with the symbol “Ph Eur” ranged left and right, respectively)。欧盟徽章中如有一个三角形,则表明该各论已被欧洲药典委员会采纳,并且通过了与《欧洲药典》、《日本药典》及《美国药典》的相关负责部门的协商,达成了一致标准(Inclusion of a triangle within the chaplet of stars denotes monographs that have been adopted by the European Pharmacopoeia Commission following their preparation according to a procedure of harmonisation agreed between the bodies responsible for the European Pharmacopoeia and those of Japan and the United States of America)。
《美国药典》由美国政府所属的美国药典委员会(The United States Pharmacopoeial Convention)编辑出版。USP于1820年出第一版,1950年以后每5年出一次修订版,从USP25版开始每年修订一次。National Formulary《国家药品集》,简称NF,1883年第一版,1980年15版起并入USP同步出版。但仍分两部分,前面为USP,后面为NF。
《英国药典》(BP)和《美国药典》(USP)全书编排大体相同,由3大部分组成。
第一部分含目录 (Contents)、序言 (Preface)、前言 (Introduction)和凡例 (General Notice)。
第二部分为各论 (Monographs),是药典的正文,记载药品 (Drugs)、制剂 (Preparations)和各种物质 (substances)的理化指标。
第三部分为附录 (Appendices),包括两项内容:1.方法 (Methods);2.说明 (Statements),是为了节省篇幅,便利检索而设的。
阅读药典时,主要读各论。但要弄清文中术语的意思和检验方法,则需翻查凡例和附录。由于篇幅有限,此处不多加介绍。
二、各论的结构 (Composition of Monograph)
各论是药典的正文,各国药典的各论大同小异。下面以《英国药典》为例,分析其结构。
(一) 题录部分内容
1.主标题 (Main Title)
2.副标题 (Subtitle)
3.化学结构式 (Chemical Structure)
4.化学式 (Chemical Formula)
5.分子量 (Molecular Weight)
6.《美国化学文摘》登记号 (The Chemical Abstracts Service Registry Number)
(二) 正文部分的内容
正文部分的内容以小标题引出,大体有如下项目。不同的物质,项目多寡不同。
1.性状 (Description)
2.溶解度 (Solubility)
3.鉴别 (Identification)
4.酸度和碱度 (Acidity and Alkalinity)
5.溶液澄明度和颜色 (Clarity and Colour of Solution)
6.氯化物含量测定 (Chloride)
7.硫酸盐含量测定(Sulphate)
8.硝酸盐含量测定 (Nitrate)
9.生物碱含量测定 (Alkaloids)
10.重金属含量测定 (Heavy Metals)
11.水分 (Water)USP干燥失重 (Loss on Drying)
12.硫酸盐灰分 (Sulphated Ash)
13.标签 (Labelling)
14.贮藏 (Storage)
15.制剂 (Preparations)
16.作用和用途 (Action and Use)
17.剂量范围 (Usual Dose Range)











 为欧盟标志,在此处表明:本各论再现《欧洲药典》。
为欧盟标志,在此处表明:本各论再现《欧洲药典》。