-
1.1《药学英语》
-
1.2总 序
-
1.3前 言
-
1.4目录
-
1.5Unit 1
-
1.5.1Text Order of the President of the People’s Republ...
-
1.5.2Supplementary Reading DRUG ADMINISTRATION LAW OF T...
-
1.6Unit 2
-
1.6.1Text A General Introduction to OTC Drugs
-
1.6.2Supplementary Reading Reading a Drug Label
-
1.7Unit 3
-
1.7.1Text Package Insert:LEXINOR 100 mg
-
1.7.2Supplementary Reading Neptunlong
-
1.8Unit 4
-
1.8.1Text Conversations Often Taking Place in a Pharmac...
-
1.8.2Supplementary Reading Making an Appointment with a...
-
1.9Unit 5
-
1.9.1Text BP 2007 Cefalexin Monohydrate(Ph Eur monograp...
-
1.9.2Supplementary Reading BP 2007 Amantadine Hydrochlo...
-
1.10Unit 6
-
1.10.1Text USP 30 Acyclovir
-
1.10.2Supplementary Reading Folic Acid
-
1.11Unit 7
-
1.11.1Text A Brief Introduction to Traditional Chinese M...
-
1.11.2Supplementary Reading Acupuncture
-
1.12Unit 8
-
1.12.1Text Importance of Influenza Vaccination for Healt...
-
1.12.2Supplementary Reading Types of Vaccines
-
1.13Unit 9
-
1.13.1Text Organization and Personnel in USA GMP
-
1.13.2Supplementary Reading Subpart G-Packaging and Labe...
-
1.14Unit 10
-
1.14.1Text Farm-to-Table Initiatives for Safer Domestic,...
-
1.14.2Supplementary Reading Sampling Produce for Pathoge...
-
1.15附录1 《中华人民共和国药品管理法》(中文版)
-
1.16附录2 《中华人民共和国药品管理法》(英文版)
-
1.17附录3 《药品说明书和标签管理规定》
-
1.18附录4 《血液制品管理条例》
-
1.19附录5 《生物制品批签发管理办法》
-
1.20附录6 《疫苗流通和预防接种管理条例》
-
1.21附录7 常用医保药品通用名、商品名、英文名对照表
-
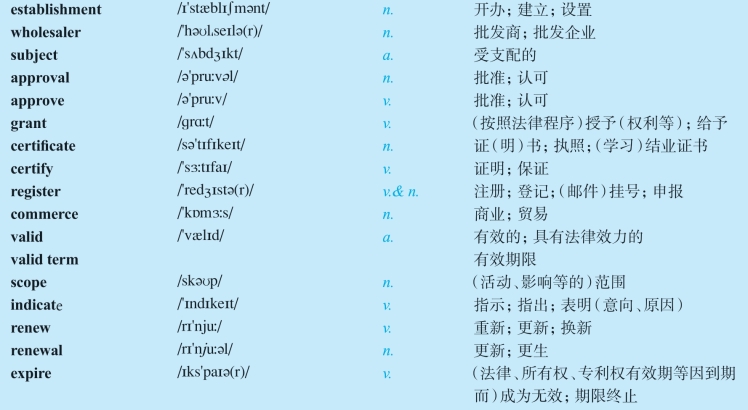
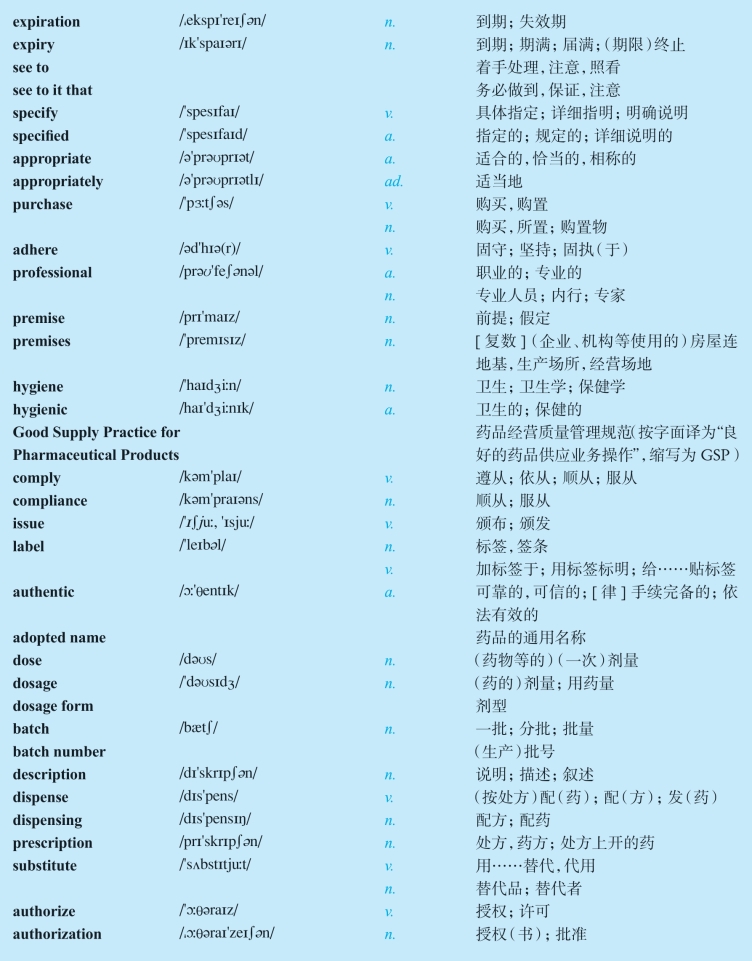
1.22Vocabulary
-
1.23汉英词汇总表
-
1.24Key to the Exercises and Scripts
1
药学英语 = Pharmaceutical English