Text Order of the President of the People’s Republic of China
(No.45)
The Drug Administration Law of the People’s Republic of China, revised at the 20th Meeting of the Standing Committee of the Ninth National People’s Congress on February 28, 2001, is hereby promulgated and shall go into effect as of December 1, 2001.
Jiang Zemin
President of the People’s Republic of China
February 28, 2001
DRUG ADMINISTRATION LAW OF THE PEOPLE’S REPUBLIC OF CHINA
(Adopted at the 7th Meeting of the Standing Committee of the Sixth National People’s Congress on September 20, 1984, revised at the 20th Meeting of the Standing Committee of the Ninth National People’s Congress on February 28, 2001)
Chapter I General Provisions
Article 1 This Law is enacted to strengthen drug administration, to ensure drug quality and safety for human beings, to protect the health of people and their legitimate rights and interests in the use of drugs.
Article 2 All institutions and individuals engaged in research, production, distribution, use, or drug administration in the People’s Republic of China shall abide by this Law.
Article 3 The State develops both modern and traditional medicines to give full play to their role in prevention and treatment of diseases and in maintenance of health.
The State protects the resources of natural crude drugs and encourages the cultivation of Chinese crude drugs.
Article 4 The State encourages research and development of new drugs and protects the legitimate rights and interests of citizens, legal bodies and other institutions engaged in this field of endeavor.
Article 5 The drug regulatory department under the State Council shall be responsible for drug administration nationwide.The relevant departments under the State Council shall be responsible for the related administrative work within the limits of their duties.
The drug regulatory departments of the people’s governments of provinces, autonomous regions, and municipalities directly under the Central Government shall be responsible for drug regulation in their administrative areas.The relevant departments of the said people’s governments shall be responsible for the related regulatory work within the limits of their duties.
The drug regulatory department under the State Council shall cooperate with the competent departments for comprehensive economic administration under the State Council in implementing pharmaceutical development programs and policies formulated by the State for the pharmaceutical industry.
Article 6 The drug testing institutes established or designated by drug regulatory departments shall undertake the responsibility for drug testing required for conducting drug review and approval and controlling drug quality in accordance with law.
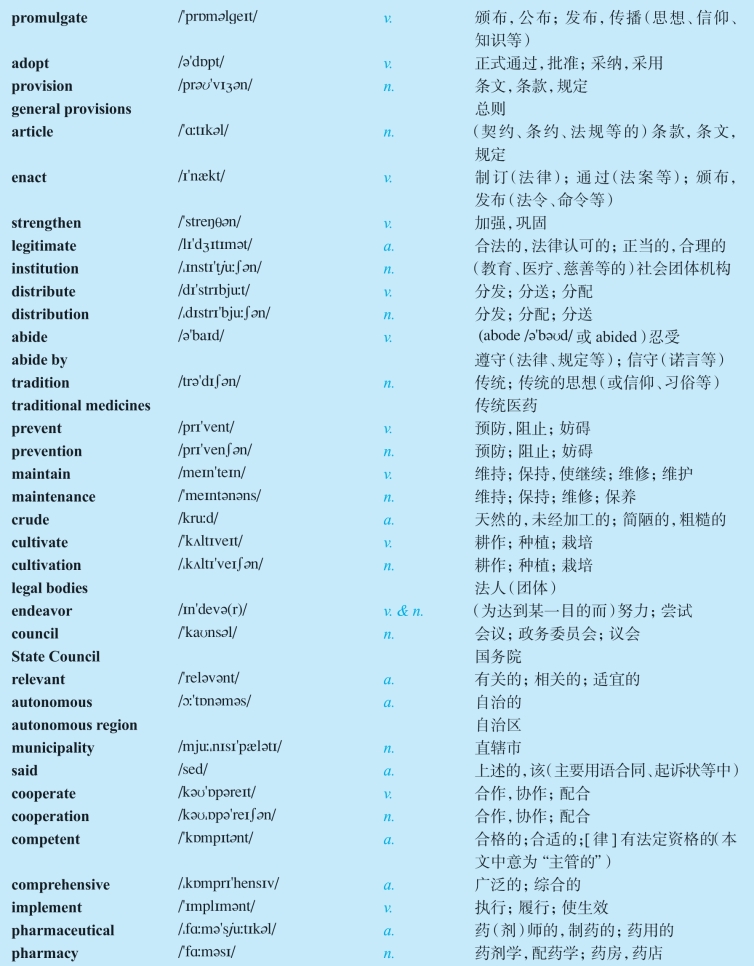
NEW WORDS AND EXPRESSIONS


NOTES
1.The Drug Administration Law of the People’s Republic of China, revised at the 20th Meeting of the Standing Committee of the Ninth National People’s Congress on February 28, 2001, is hereby promulgated and shall go into effect as of December 1, 2001.
句中 The Drug Administration Law of the People’s Republic of China ...is hereby promulgated and shall go into effect as of December 1, 2001.是整个句子的主要成分,译为:《中华人民共和国药品管理法》特此公布,并于2001年12月1日生效。
go into effect:生效
两个逗号之间的是分词短语,代替了一个非限制性定语从句,相当于which was revised at the 20th Meeting of the Standing Committee of the Ninth National People’s Congress on February 28, 2001,译为:该法已在2001年2月28日全国人民代表大会常务委员会上通过修订。
2.Adopted at the 7th Meeting of the Standing Committee of the Sixth National People’s Congress on September 20, 1984, revised at the 20th Meeting of the Standing Committee of the Ninth National People’s Congress on February 28, 2001.
该段落由两个分词短语组成,第一个分词短语adopted ...是主要成分,相当于The Law was adopted at ...,译为:该法于……被通过;第二个分词短语revised ...是并列谓语,相当于 and it was revised at ...,译为:该法于……通过后,又在……经过修正。
3.This Law is enacted to strengthen drug administration, to ensure drug quality and safety for human beings, to protect the health of people and their legitimate rights and interests in the use of drugs.
This Law is enacted 后连续用了 to strengthen, to ensure, to protect 3个不定式短语,表示立法的目的,译为:为了加强……;为了保证……;为了维护……
4.All institutions and individuals engaged in ...
engaged in 是一个分词短语,相当于All institutions and individuals that are engaged in ...
be engaged in=be involved in (with):从事……
5.The State develops both modern and traditional medicines to give full play to their role in prevention and treatment of diseases and in maintenance of health.
The State develops both modern and traditional medicines ...:国家既发展现代药学,也发展传统药学。
develop both ...and ...:既发展……也发展……
to play to one’s role in:在……中发挥作用
6.citizens, legal bodies and other institutions engaged in this field of endeavor:从事此项工作的(指前述的研究和开发新药)公民、法人和其他机构
field意为“领域”,endeavor意为“努力”。
7.The drug regulatory department under the State Council shall be responsible for drug administration nationwide.
The drug regulatory department under the State Council:国务院下属的药品监管部门
句中under 是介词,意思为“在其下的”。
be responsible for:对……负责,承担……的责任
全句可译为:国务院下属的药品监督管理部门,主管全国的药品监督管理工作。
8.the related administrative work within the limits of their duties
the related administrative work: 有关的监督管理工作
within the limits of their duties: 在他们各自的职责范围内
9.The relevant departments of the said people’s governments shall be responsible for the related regulatory work within the limits of their duties.
The relevant departments of the said people’s governments:上述人民政府的相关部门
全句可译为:上述(即省、自治区、直辖市)人民政府的有关部门应在各自的职责范围内负责(与药品)有关的监督管理工作。
10.The drug regulatory department under the State Council shall cooperate with the competent departments for comprehensive economic administration under the State Council in implementing pharmaceutical development programs and policies formulated by the State for the pharmaceutical industry.
cooperate with the competent departments:与主管部门合作;配合主管部门
for comprehensive economic administration 是介词短语,后置做定语,修饰 departments for comprehensive economic administration (负责经济综合管理的部门)。
in implementing pharmaceutical development programs and policies formulated by the State for the pharmaceutical industry 是介词短语做状语,表示配合的领域,译为:(在)执行国家制定的药品行业发展规划和政策(领域、方面)。
11.The drug testing institutes established or designated by drug regulatory ...departments established or designated by drug regulatory departments 是两个并列的过去分词短语代替定语从句,修饰the drug testing institutes (药品检验机构)。完整的句子是:The drug testing institutes (that were)established or designated by drug regulatory departments 由药品监督管理部门设置或指定的药品检验机构
12....undertake drug testing required for conducting drug review and approval and controlling drug quality in accordance with law.
required for conducting drug review and approval 是分词短语,代替定语从句修饰 drug testing (药品检验)。
...undertake drug testing (which is)required for conducting drug review and approval and controlling drug quality,译为:……承担药品审批和药品质量控制所需的药品检验工作。
in accordance with law=according to law 依据法律
Exercises
Task 1 Choose A, B or C to complete each statement below according to the text.
1.The Drug Administration Law of the People’s Republic of China came into effect on __________.
A.February 28, 2001 B.December 1, 2001 C.September 20, 1984
2.__________ are engaged in research, production, distribution, use, or drug administration in the People’s Republic of China shall abide by this Law.
A.All those that B.All those C.Everybody who
3.The drug regulatory department under the State Council shall ___________ drug administration nationwide.
A.take responsible for B.take responsible to C.take the responsibility for
4.The drug regulatory department under the State Council shall cooperate with the competent departments for comprehensive economic administration under the State Council __________ pharmaceutical development programs and policies formulated by the State for the pharmaceutical industry.
A.in order to carry out B.in order that C.so as to meet
5.The drug testing institutes established or designated by drug regulatory departments shall undertake the responsibility for drug testing __________ for conducting drug review and approval and controlling drug quality in accordance with law.
A.requiring B.to be require C.that is required
Task 2 Translate the following into Chinese.
Article 7 The establishment of a drug manufacturer1shall be subject to approval by the local drug regulatory department of the people’s government of the province, autonomous region or municipality directly under the Central Government and be granted the Drug Manufacturing Certificate, and, with the certificate, the manufacturer shall be registered with the administrative department for industry and commerce.No one may manufacture drugs without the certificate.
The valid term and the scope of manufacturing shall be indicated in the Drug Manufacturing Certificate.For renewal of the certificate on expiration, reexamination2is required.
When giving approval to the establishment of a new manufacturer, the drug regulatory department shall see to it that, apart from the requirements specified by the provisions in Article 8 of this Law that should be met, the pharmaceutical development programs and policies formulated by the State for the pharmaceutical industry are conformed to and duplicate3construction is prevented.
Notes:1.manufacturer 制造企业 2.reexamination 重新审查 3.duplicate 重复的
法律英语的文体特点
法律英语是以英语共同语为基础、在立法和司法等活动中形成和使用的具有法律专业特点的语言。因此,在法律英语中不仅有众多的具有法律专门意义的特殊词汇,而且由于规定人们权利和义务的法律、法令或契约等法律文书所表述的内容必须准确、严密、客观和规范,不容许丝毫的引申、推理或抒发和表达感情,因而在法律英语中又形成了许多其特有的句法特点,这些词法和句法特点在翻译过程中必须受到充分重视。下面我们从词汇、词类使用和句型结构三方面来考察法律英语的文体特点:
一、法律英语的文体特点之一:法律英语词汇
1.用词:庄重、规范、书面语较多
法律是掌握国家政权的阶级、集团的意志体现,它有鲜明的政策性,权威性。为了维护法律的严肃性,法律、法规遣词造句力求准确,用词正式,语意严谨。不像文学作品那样,有华丽的辞藻和丰富的修饰语,也不可能使用比喻、夸张和委婉语气。
Article 1 This Law is enacted to strengthen drug administration, to ensure drug quality and safety for human beings, to protect the health of people and their legitimate rights and interests in the use of drugs.
第一条 为加强药品监督管理,保证药品质量,保障人体用药安全,维护人民身体健康和用药的合法权益,特制定本法。
本句的enact,legitimate等词都是非常正规的书面语,没有任何修饰或夸张成分。
2.运用成对词和近义词
在各种法律条文中,我们可以常见到以下的类似用法:rights and interests (权益),institutions and individuals (团体与个人),research and develop (研究与开发),both modern and traditional (现代与传统的),sign and issue (签发),这些词表示固定的意义,使用和翻译时不能随意拆开。
3.大量使用命令词和情态动词
由于法律,法规代表统治阶级的意志,表现司法主体对司法客体的行为制约和义务规定,它通常要求司法客体“必须”、“可以”、“应该”或“不许”、“不能”、“不得”做什么,用词通常带命令语气。
读者可以在本课及补充阅读部分的各条款中看shall重复了22次,may重复4次,should使用了一次。
二、法律英语的文体特点之二:词类的使用特点
在词类的选用上,法律英语也有其独特之处。
1.代词
由于法律条文的严密性,因而对代词的使用非常谨慎,尽可能少地使用代词,尤其是指示代词、不定代词等。无人称代词it在法律英语中除用于作形式主语、宾语的语句中外,一般少用。而普通英语正好相反,为了避免词的重复出现或使句子更加简洁,常多用代词。《中华人民共和国药品管理法》中,the drug regulatory department under the State Council(国务院药品监督管理部门)等名词多次重复,而不像在普通问题中,第一次出现时用名词,再次出现一般要用代词。
2.名词
法律英语的名词复数一般都是规则变化,很少出现不规则变化的名词复数。在法律条文中抽象名词居多。当名词用作主语或宾语的中心词时,其限定词语多。因而名词在法律英语中所出现的频率比其他任何词性所出现的频率都高,甚至在有些条文中不用动词,只用名词短语来表述其法律条文或法律概念。请看以下例句:
Article 15 A drug distributor to be established shall meet the following requirements:
(1)having legally qualified pharmaceutical professionals;
(2)having the business operation premises, equipment, warehouses and hygienic environment required for drug distribution;
(3)having the units or personnel for quality control over the drugs to be distributed; and
(4)having rules and regulations to ensure the quality of the drugs to be distributed.
此处连续用了4个动名词短语。
3.动词
法律语言的社会功能使得法律英语动词使用的语气、语态与时态与普通英语有所区别。法律的强制性使得祈使语句在法律英语很普遍,shall, may, must, be to的使用频率很高。法律的施动性使得被动语态在法律英语中广为使用,在正式的法律法规的法律英语中,一般现在时、现在完成时和一般将来时用得比较多,而案例和律师陈述中,一般过去时用得比较多。
4.形容词和副词
由于法律英语文体要求其语言多为客观描述性与解释性,所以很少使用表示程度强烈的形容词和副词,尤其是very, quite, rather极为少用。
三、法律英语的文体特点之三:句法结构特点
1.陈述句的使用
众所周知,根据句子使用目的的不同,英语句子可以分为陈述句、疑问句、祈使句和感叹句四大类。由于法律文书是用来确认法律关系、贯彻法律条令、规定人们的权利和义务以及陈述案件事实的专用公文,所以法律英语的基本句式通常是陈述句结构。
2.完整句的使用
由于法律文书结构的完整性和表意的严密性,在法律英语句子的使用中,一般采用主语、谓语都具备的完全主谓句,即完整句,通常不使用省略句或单部句,以免造成因省略或句子缺省而出现歧义讹误,甚至被人任意歪曲。
3.长句的使用
法律英语的句法特点是和法律英语的文体特征密切相连的。正式的法律条规和文本中由于对中心词的限定过多,对某一法律概念成立的条件限定很多,所以法律英语的长句居多,短句少,引语少。
4.状语分句的使用
法律文书中关于义务部分的陈述是至关重要的,它是享受权利的前提和条件。在法律英语中关于义务的陈述表现在句子结构上,往往使用条件状语分句或让步状语分句,从而成为法律英语长句多的主要原因。
5.关系分句的使用
法律英语中长句的形成不仅来源于句首冗长而复杂的状语分句,也体现在句子内部起修饰、限制作用的各种关系分句中。
6.综合复杂句的使用
法律英语中除了一方面在句首使用状语分句,另一方面在句子的内部使用层层限制修饰的关系分句外,还大量地使用由状语分句和关系分句以及其他一些分句构成的综合复杂句,以达到准确、严密和不产生歧义的目的。
7.定语从句
法律英语较之普通英语更准确,限定更严谨,所以限定性定语从句这一必不可少的定语运用得比较普遍,而作为补充、说明、解释性的非限制性定语从句用得比较少。
8.条件状语从句与虚拟语气
法律英语的正式条文法规中,一般只采用有条件的、符合逻辑推理的、能出现或产生真实结果的条件状语从句,而很少使用虚拟语气的句子。
9.被动句的使用
法律英语句子中大量地使用被动句,这是由法律文书的客观性所决定的。在法律英语中使用被动句正是实现这一目的的一个重要手段。所以,在法律英语中被动句的使用真可谓缤纷繁多。例如:仅在第14条中出现的被动语态就有如下几处:
...the wholesaler and the retailer shall be registered with the administrative department for industry and commerce.No one may distribute drugs without the certificate.
The valid term and the scope of business shall be indicated in the Drug Supply Certificate.For renewal of the certificate upon expiration, reexamination is required.
...the requirement specified by the provisions in Article 15 of this Law that should be met, ...
10.时态的使用
通常法律英语在时态的使用上一方面大多采用一般现在时,用以阐述道理、确立规范或规定权利、义务等;另一方面在表示“应当、必须”等义务或职责的法律规定时,在第三人称主语后使用shall和be to结构。


