Chapter 13 Grignard Reagent
Francois Auguste Victor Grignard was born in Cherbourg, France on May 6, 1871. From 1883 to 1887 he attended local schools, and in 1889 he was awarded a scholarship to the Ecole Normale Special at Cluny, which was a school intended to produce teachers for modem secondary schools. But in 1891 the school was closed because supporters of the “classic” methods of secondary education and those of“modem” ones had a dispute. In order to finish the entitlement of their scholarships, Grignard was transferred with his classmates to other establishments. Fortunately, Grignard himself joined the University of Lyons, and he was attached to the Faculte Sciences. However, he was not successful in the licentiate examination in mathematics. In 1892 he left to join the military and he was demobilized at the end of 1893. He then returned to Lyons to continue his study. In 1894 he gained the degree Licencie of Sciences Mathematiques.
After studying mathematics he worked as a chemistry technician at the University of Lyons. Initially he had a bad impression about chemistry as a discipline inferior to mathematics. He described chemistry as purely empirical. He changed his opinion after doing some laboratory work, and began his graduate studies at the University of Lyons under Philippe A. Barbier, one of the pioneers in developing the chemistry of compounds that contain a carbon-metal bond. In 1899 Barbier had discovered that magnesium could be used in place of zinc to promote the reaction of methyl iodide with a ketone to give a tertiary alcohol. This represented a useful advance in organometallic chemistry because the zinc reagents that were pioneered by chemists A. M. Zaitsev of Russia and E. Frankland of Britain, and being used at the time, could not yield satisfactory results in many cases. Barbier encouraged young Grignard to further explore reactions of magnesium with alkyl halides for his doctoral research project. Grignard was a conscientious, hard-working student with modesty and a friendly disposition.
Grignard scrutinized the literature on organozinc compounds and organomagnesium compounds. He noticed Frankland’s papers published in 1850s, which mentioned that organozinc compounds were stable in anhydrous ether and nonflammable in air. This prompted Grignard to test the method of slowly adding to magnesium of a mixture of isobutyl iodide and anhydrous ether. On cooling, the product was a clear, colorless liquid that was stable in air. More interestingly, after addition of benzaldehyde, the solution generated a high yield of phenyl isobutyl methanol. The reaction occurred spontaneously at room temperature. He also quickly discovered that a wide variety of alkyl and aryl halides reacted readily with magnesium in anhydrous ether to give the corresponding alkyl- and arylmagnesium halides that are now known as Grignard reagents. His initial results were reported in 1900, and he published seven additional papers within the next year on the preparation of organomagnesium compounds and their reactions with carbonyl compounds to give alcohols. After receiving his Ph. D. degree in 1901, Grignard continued his research at the University of Lyons and then the University of Nancy where he explored the scope and limitations of the reactions of Grignard reagents. During this time, he discovered that the reactions that now bear his name could be used to prepare primary, secondary, and tertiary alcohols, ketones, esters, and carboxylic acids.
Grignard Reaction
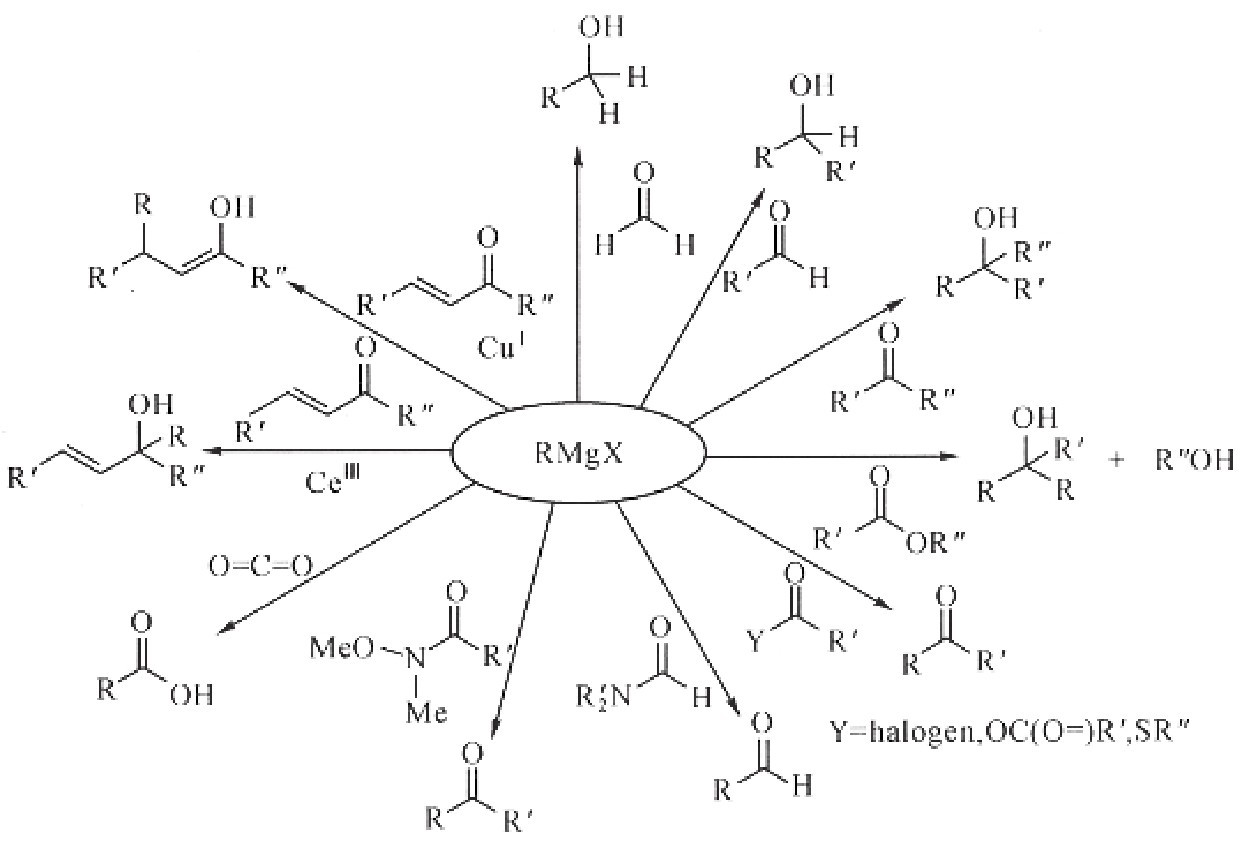
The discovery and development of the chemistry of Grignard reactions were especially important for applications to the synthesis of complex molecules because a new carbon-carbon bond is formed by combining two simple building blocks, namely an alkyl or aryl halide and a carbonyl compound. The products of Grignard reactions also contain functionality that may then be used in subsequent transformations to fashion even larger molecules. It occurs in two steps:
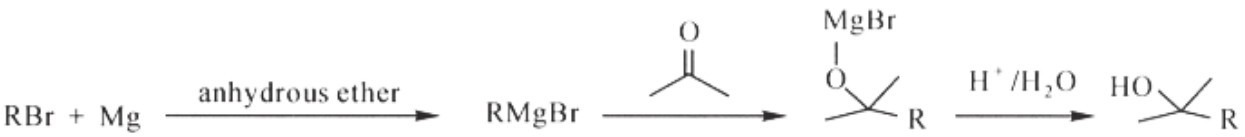
1. Generation of the “Grignard reagent”, which is an organomagnesium compound formed by the reaction of Mg with an organohalide, R-X (R is an alkyl or an aryl and X is a halide, usually bromide or iodide). Typically, the Grignard reagent is represented by the general chemical formula RMg X. However, structure of the Grignard reagent is more complex. In order to stabilize a Grignard reagent,nonbonded electrons from the oxygen in an ether solvent are required.
2. Addition of the carbonyl. During this step, a ketone or an aldehyde is added to the solution with the Grignard reagent. The carbon atom bonded to magnesium transfers to the carbonyl carbon atom, while the oxygen of the carbonyl is attached to magnesium and affords an alkoxide. Such a process is an example of a nucleophilic addition to a carbonyl. After the addition, the addition of aqueous acid to the reaction mixture offers an alcohol, followed by the discarding of the magnesium salts.
For this work, Grignard won the Nobel Prize in Chemistry in 1912 together with fellow Frenchman Paul Sabatier. With regard to this prize, Grignard said that the Nobel committee was unfair and he should receive this prize jointly with his mentor Barbier for whom he worked for 14 years. He returned to the University of Lyons in 1919 where he succeeded Barbier as head of the Department and continued his research. Grignard also conducted an extensive research program in the field of terpenoid natural products, ozonolysis, aldol reactions, and catalytic hydrogenation and dehydrogenation, and in WWI he was responsible for development of chemical weapons of mustard gas and phosgene for which he was criticized. However, it is for his development of the chemistry of Grignard reagents that he is best remembered. Indeed, at the time of his death in 1935, more than 6000 papers had appeared in which applications of Grignard reactions were described.
Reaction Mechanism
The addition of the Grignard reagent to the carbonyl usually proceeds via a six-membered ring transition state, although with hindered Grignard reagents, the reaction can possibly proceed through single-electron transfer. For reactions involving Grignard reagents, it is necessary to ensure an anhydrous environment; otherwise, water would cause the reagent to rapidly decompose. Therefore, most Grignard reactions take place in solvents such as anhydrous diethyl ether or tetrahydrofuran, because the magnesium reagent is stabilized by the oxygen of these solvents. It is also possible for the reagent to react with oxygen present in the atmosphere, in which an oxygen atom inserts between the carbon base and the magnesium halide group. Usually, using the volatile solvent vapors to displace air above the reaction mixture may limit this side-reaction, although preferably such reactions may be carried out in nitrogen or argon atmospheres, especially for smaller scales.
Preparation of Grignard Reagent
The preparation of Grignard reagent is very simple. There is only one essential precaution to take: the operation must be carried out in a medium free from moisture and atmospheric oxygen.
The apparatus comprises a spherical flask that is surmounted by a good reflux condenser and a bulb with a tap. One mol of magnesium (24 g) is placed in the flask, and 1 mol of methyl iodide is dissolved in an equal volume of anhydrous ether. By means of the tap 25-30 cubic centimeters of this solution are allowed to drop from the bulb on to the magnesium. A very intense reaction occurs almost immediately. And it is moderated by adding, all at once, 200-250 cubic centimeters of anhydrous ether to the flask; then the rest of the reaction mixture is allowed to drip on to the magnesium to maintain the reaction. The operation is completed by heating for a few moments, if necessary. Under these conditions all the magnesium disappears and we finally get a perfectly fluid and colorless liquid, although at first it shows a slate-colored cloudiness due to extremely minute particles of iron from a slight impurity in the magnesium used.
Coupling Reactions
Some coupling reactions can also involve a Grignard reagent. For example, nonylmagnesium bromide may react with an aryl chloride to a nonyl benzoic acid when iron (III) acetylacetonate is present. Ordinarily, the Grignard reagent attacks the ester over the aryl halide. For the coupling reactions of aryl halides with aryl Grignards, one of the effective catalysts is nickel chloride in THF. In addition, a good catalyst for the couplings of alkyl halides is dilithium tetrachlorocuprate (Li2Cu Cli4), which is prepared by mixing lithium chloride (Li Cl) and copper (II) chloride (Cu Cl2) in THF. The Kumada-Corriu coupling reaction with the Grignard reagent gives access to styrenes.
The Grignard reagent is active. Water violently decomposes it; it fixes oxygen and carbon dioxide gas, and it also reacts vigorously with almost all the organic functional groups that we know in organic chemistry. However, one advantage it already possesses over the organic zinc compounds is that it can be safely exposed to air without the danger of igniting. It is therefore no more dangerous to handle than ether itself.