In-Class Design - "IUPAC Nomenclature: The 'Chemical UN' for Molecules"
Isobutane vs. 2-Methylpropane; Citral (common name) vs. 3,7-Dimethyl-2,6-octadienal (IUPAC).
Before unified rules, the chemical world was chaotic. Today, your mission, diplomats, is to master this universal language—IUPAC Nomenclature—to describe any molecule accurately and unambiguously.
Training Camp.
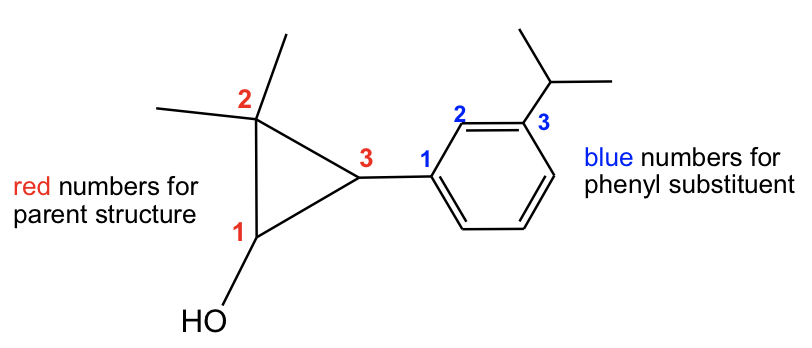
2,2-dimethyl-3-(3-isopropylphenyl)cyclopropanol
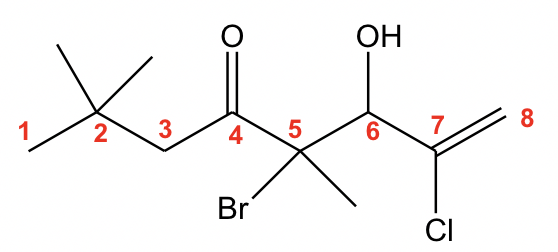
With the multiple groups involved, the ketone has the highest priority, so it decides the last name. The 8-carbon alkene chain with ketone should be name as “octenone”. The numbers on the chain should start from the left side to ensure that ketone has the lowest number. When the OH group is regarded as a substituent, it is indicated by the prefix “hydroxy”. So the complete name is “5-bromo-7-chloro-6-hydroxy-2,2,5-trimethyl-7-octen-4-one”.
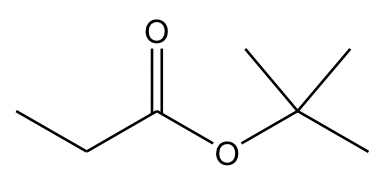
In ester, an OR group replaces the OH group of a carboxylic acid. When naming the ester, the name of the R in the OR group is stated first, followed by the name of the acid, with “oic acid” replaced by “oate”. As a net result, the R in the OR is regarded as the “substituent”, even though it is not. So, the complete name of the ester above is “tert-butyl propanoate”.
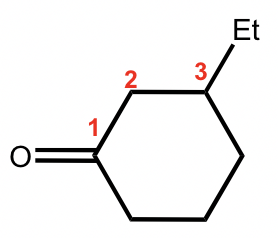
This is a ketone based on a cycloalkane, so the last name comes from “cyclohexane’. By adding the suffix, it become “cyclohexanone”, and the complete name is “3-ethylcyclohexanone”.
Trivial Names
Investigation: Choose 3 organic compounds with well-known trivial/trade names (e.g., Aspirin, Acetone, Glucose, Camphor).
Draw their complete structural formulas.
Provide their systematic names according to IUPAC rules.
Research and explain the historical origin of their trivial names (e.g., from Latin, discovery process, source). E.g., "Acetone" derives from "acetic acid."
Reflection: Write a short paragraph discussing: Even though the IUPAC system is more precise, why are these trivial names still widely used today? What does this reveal about the realities of scientific communication?
Design Brief: You are the lead chemist at a pharmaceutical or fragrance company. Design a core molecule for a new product you are developing (a targeted drug or a new perfume).
Design Constraints:
The molecule must have a main chain of at least 6 carbon atoms.
It must contain at least 3 different functional groups (e.g., -OH, -CHO, -NH₂, benzene ring, etc.).
The structure cannot be identical to any well-known, common molecule.
Deliverables:
Blueprint: Draw the structural formula of your designed molecule on paper or using chemical drawing software.
Official Dossier: Provide the complete and correct IUPAC name for this molecule.
Creative Marketing: Give your molecule a trade name and write a marketing tagline (max 100 words) explaining how the trade name reflects its structure or function.
提交形式/Deliverable:
一份结合了研究报告与创意设计的综合文档/作品集。
A comprehensive document/portfolio combining the research report and the creative design.


