Chapter 1 The nature of chemistry
辩证唯物主义世界观 Science of matter
可持续发展的社会责任 Green Chemistry and Sustainable development
实事求是与探索真理的科学精神 Experimental science
Serve the people, contribute to the country and safeguard the home of mankind.
认识世界思维方式与价值取向 Understand the world
改造世界的知识和能力Transform the world
1.
The nature of chemistry
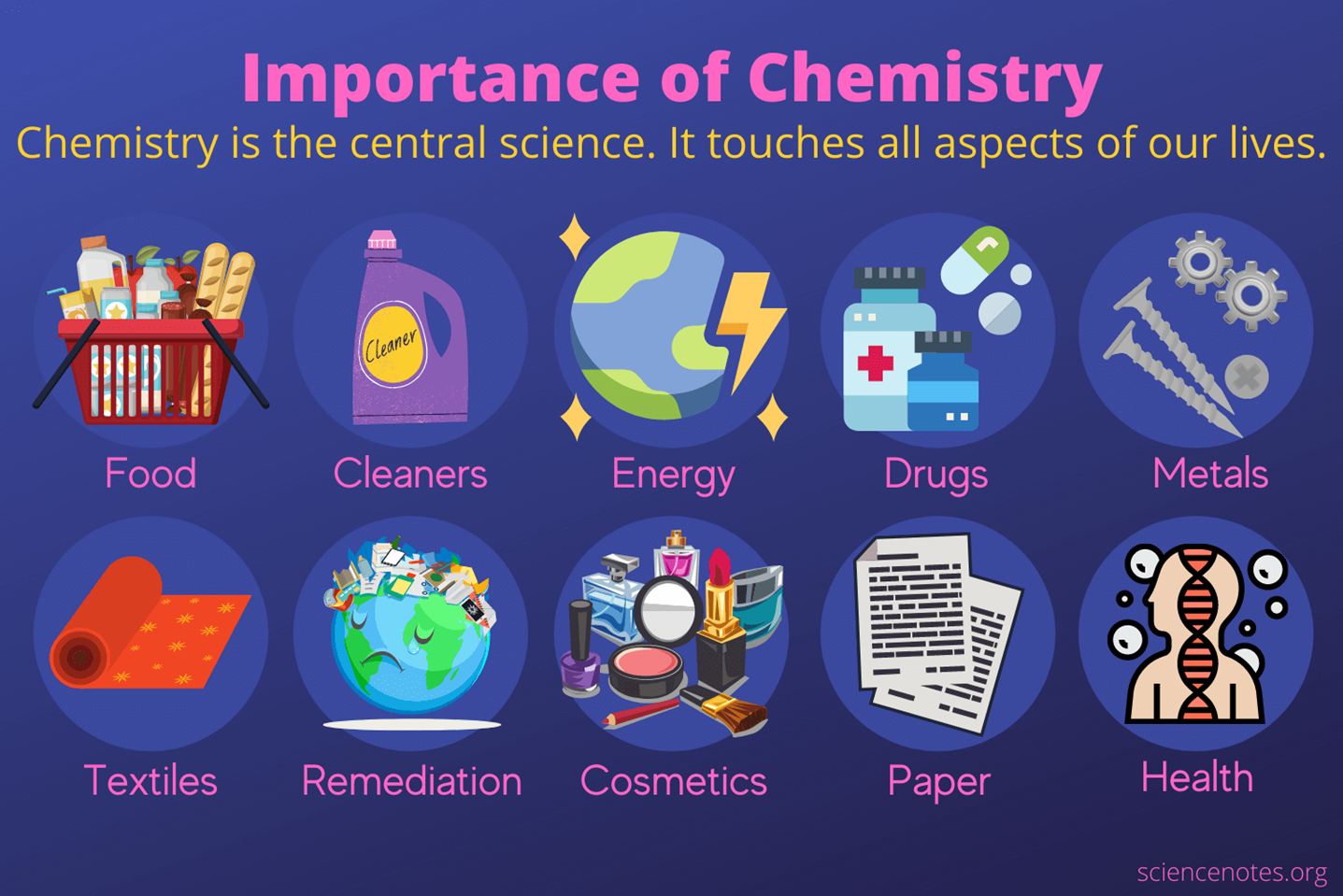
Chemistry, often called the “central science,” is the branch of science that deals with the composition, structure, properties, and changes of matter. It connects the other natural sciences, including physics, biology, and geology, by explaining how substances interact and transform.
At its core, chemistry is the study of matter. This means understanding what matter is made of, how it behaves, and what happens when it changes. Matter is everything around us: the substances that make up the air, the earth, the oceans, and all living things. It exists in various states—solids, liquids, gases—and changes in response to different conditions.
The study of chemistry goes beyond just understanding the properties of matter. It also delves into the processes and reactions that occur when substances interact with one another. These reactions are at the heart of chemical changes, which are essential for everything from biological processes in our bodies to the way industrial products are made.
Chemistry is one of the physical sciences.
Chemistry is the science of matter.
Chemistry is an experimental science.
2. Branches of chemistry
Organic chemistry, inorganic chemistry, analytical chemistry, physical chemistry, and biochemistry are the main branches of Chemistry, but it is possible to combine portions of them, or to elaborate on them in many ways.
Organic Chemistry: Organic chemistry is the study of carbon and its compounds. It is the study of the chemistry of life and reactions occurring in living organisms. An organic chemistry student might study organic reactions, the structure and properties of organic molecules, polymers, drugs, or fuels.
Inorganic Chemistry: Inorganic chemistry is the study of compounds not covered by organic chemistry. It is the study of inorganic compounds or compounds that don't contain a C-H bond. A few inorganic compounds do contain carbon, but most contain metals. Topics of interest to inorganic chemists include ionic compounds, organometallic compounds, minerals, cluster compounds, and solid-state compounds.
Analytical Chemistry: Analytical chemistry is the study of the chemistry of matter and the development of tools to measure the properties of matter. Analytical chemistry includes quantitative and qualitative analysis, separations, extractions, distillation, spectrometry and spectroscopy, chromatography, and electrophoresis. Analytical chemists develop standards, chemical methods, and instrumental methods.
Physical Chemistry: Physical chemistry is the branch of chemistry that applies physics to the study of chemistry, which commonly includes the applications of thermodynamics and quantum mechanics to chemistry.
Biochemistry: Biochemistry is the study of chemical processes that occur inside living organisms. Examples of key molecules include proteins, nucleic acids, carbohydrates, lipids, drugs, and neurotransmitters. Sometimes this discipline is considered a subdiscipline of organic chemistry. Biochemistry is closely related to molecular biology, cell biology, and genetics.
Electrochemistry: Electrochemistry examines the movement of charge in chemical systems. Often, electrons are the charge carriers, but the discipline also investigates the behavior of ions and protons.
Green Chemistry: Green chemistry looks at ways of minimizing the environmental impact of chemical processes. This includes remediation as well as ways of improving processes to make them more eco-friendly.
Geochemistry: Geochemistry examines the nature and properties of geological materials and processes.
Nuclear Chemistry: While most forms of chemistry mainly deal with interactions between electrons in atoms and molecules, nuclear chemistry explores the reactions between protons, neutrons, and subatomic particles.
Polymer Chemistry: Polymer chemistry deals with the synthesis and properties of macromolecules and polymers.
Quantum Chemistry: Quantum chemistry applies quantum mechanics to model and explore chemical systems.
Radiochemistry: Radiochemistry explores the nature of radioisotopes, the effects of radiation on matter, and the synthesis of radioactive elements and compounds.
Theoretical Chemistry: Theoretical chemistry is the branch of chemistry that applies mathematics, physics, and computer programming to answer chemistry questions.


