2.2 Differences between theThree States of Matter
¡We will first show how these states differ physically from one another.
A. The Gaseous State
¡Definition— a state of matter in which the substance expands readily to fill any containing vessel.---What this means is that any collection of molecules in the gaseous state is free to move in all directions and that the gaseous molecules will fill any container in which they are confined.
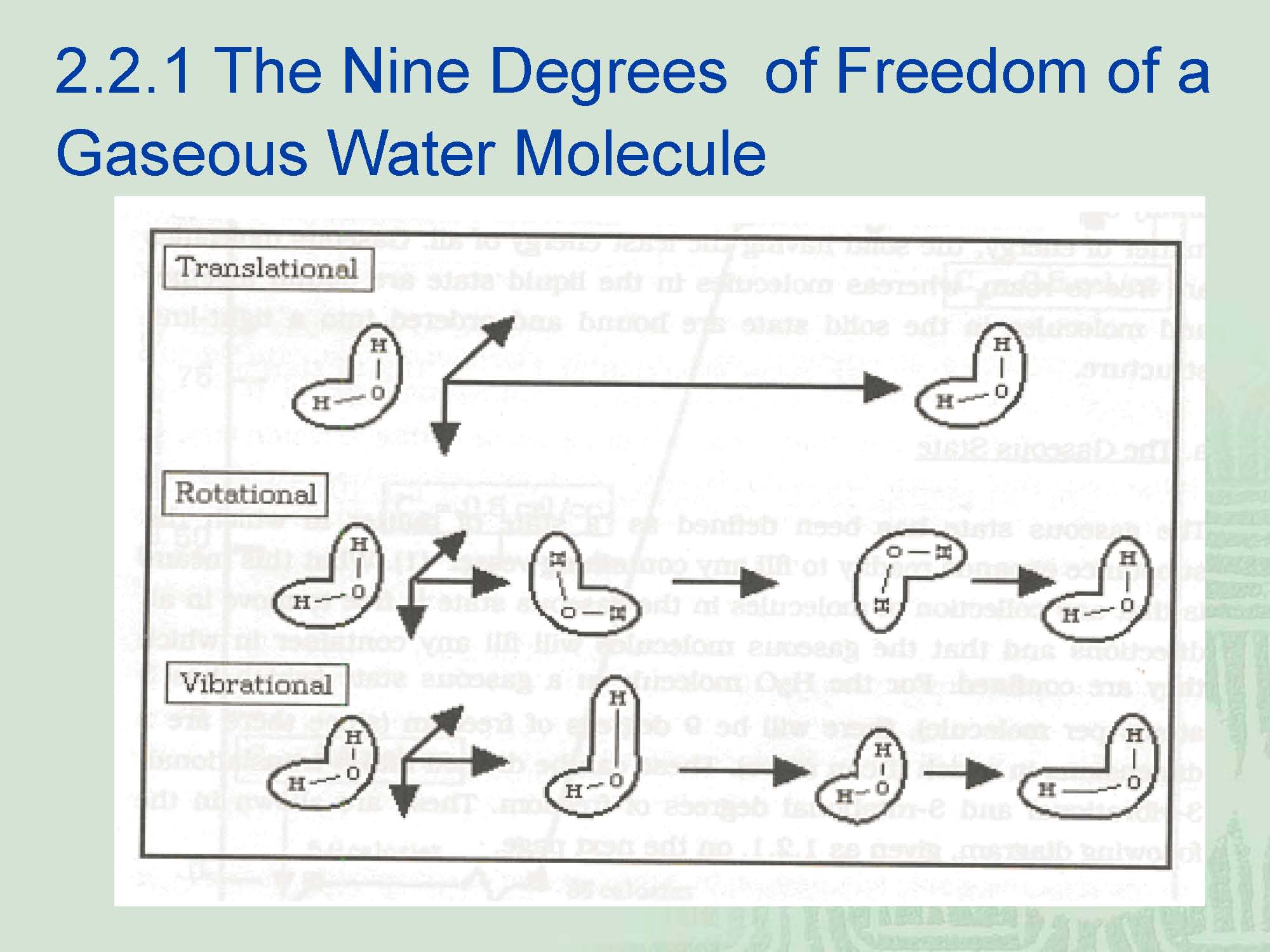
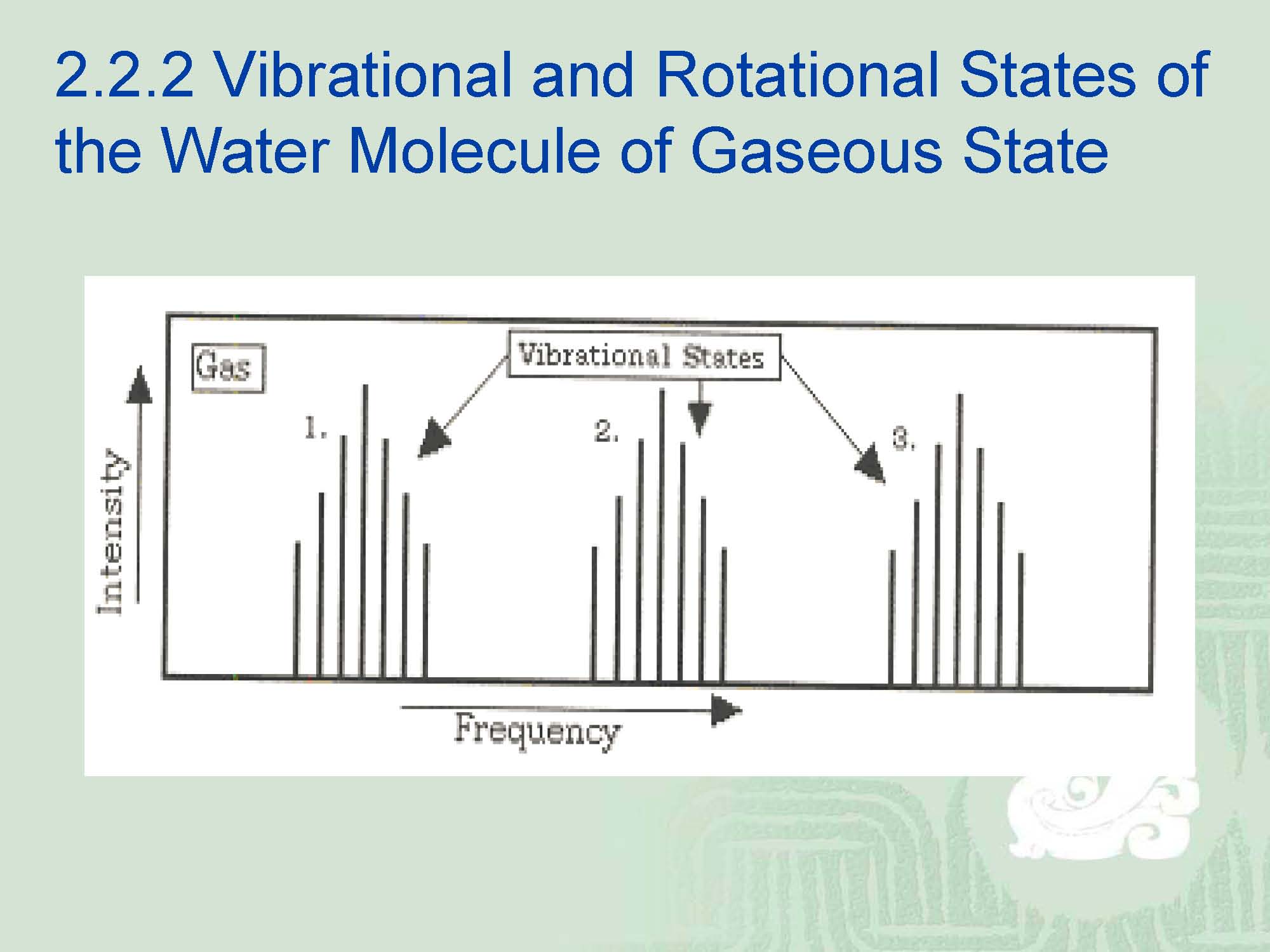
¡For the gaseous water molecule which has three atoms per molecule, the 3 vibrational degrees of freedom will have 7(2J+1=7) rotational states superimposed upon them.
¡If the molecule happened to be NH3, then the expected number of vibrational states would be nine.
¡The exact region of the spectrum depending on the type of molecule present(i.e.-molecular weight).
¡Mean free path– the average distance that each molecule moves before collision. It is a function of both the temp. and the pressure of the gas. –This concept arose from the Kinetic Theory of Gases which in turn arose from Avagadro’s Hypothesis.
¡It was Maxwell in 1848 who showed that molecules have a distribution of velocities and that they do not travel in a direct line.
¡Avogadro’s Number, 6.02204531x1023----Perrin and Einstein worked
B. The Liquid state
¡In the liquid state, the molecules are still free to move in three dimensions but still have to beconfined in a container in the same manner as the gaseous state if we expect to be able to measure them.
¡The important differences between liquid andgas----there are energy removed from liquid molecule in order to get them to condense. The translational degrees of freedom are found to be restricted.—the molecules are much closer together and interact with one another.
¡Result—The molecules of a liquid are not free to flow in any of the three directions, but are bound by intermolecular forces.
¡For example, H—O—H : Dipole
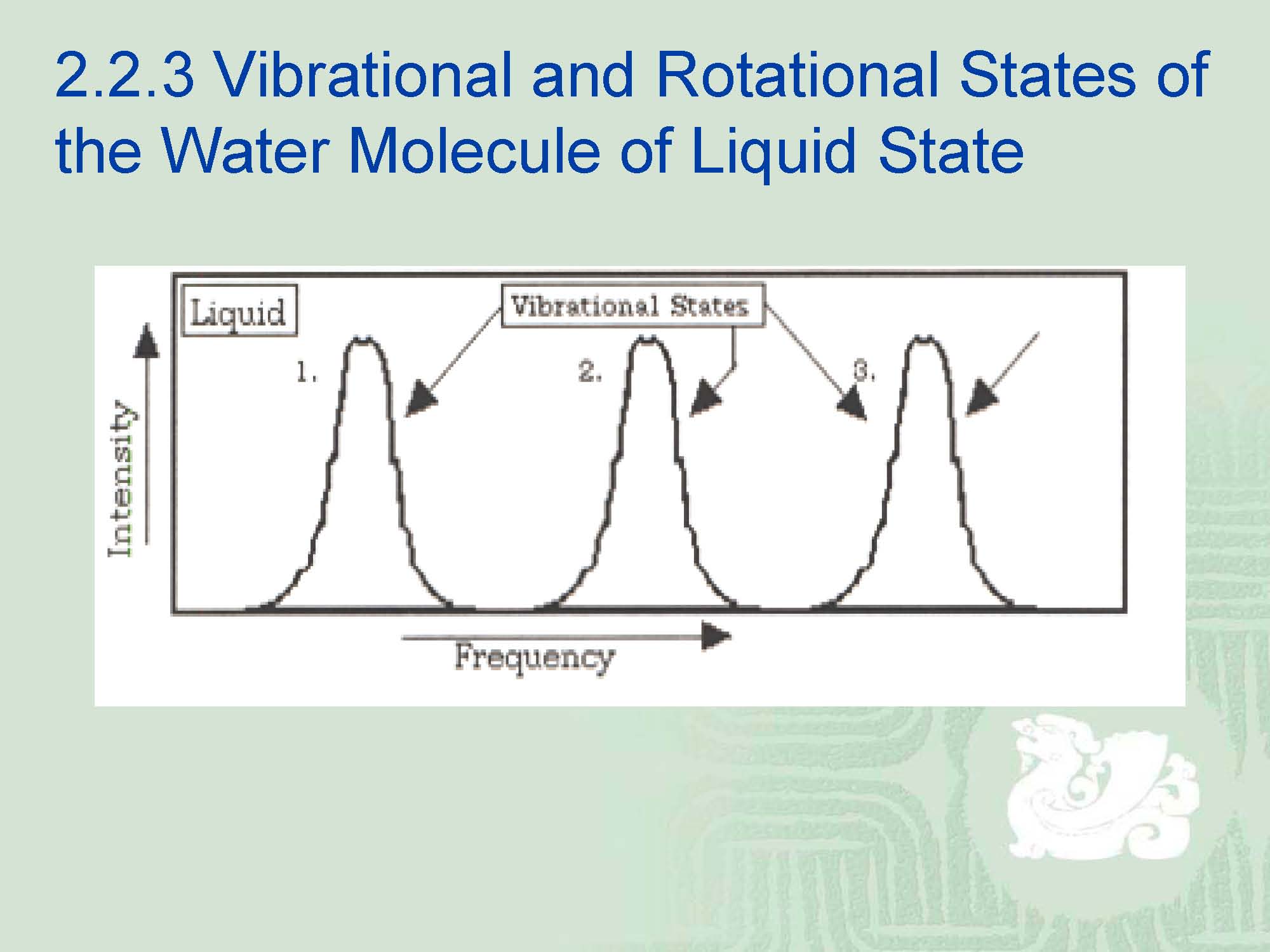
¡Note that in the above diagram there are still vibrational states but that the rotational states are “smeared” one into other. There is little translational motion for the water molecules within the interior of the liquid unless they escape from the liquid phase.
¡Evaporation---L-G
¡Sublimation---S-G
¡Most liquids do have a defined vapor pressure which means that molecules can and do escape from the surface of the liquid to form agas.
¡This is another reason that the properties of a liquid vary from those of the gaseous state.
¡Hence,we still have the vibrational and rotational degrees of freedom left in the liquid, but not those of the translational mode.
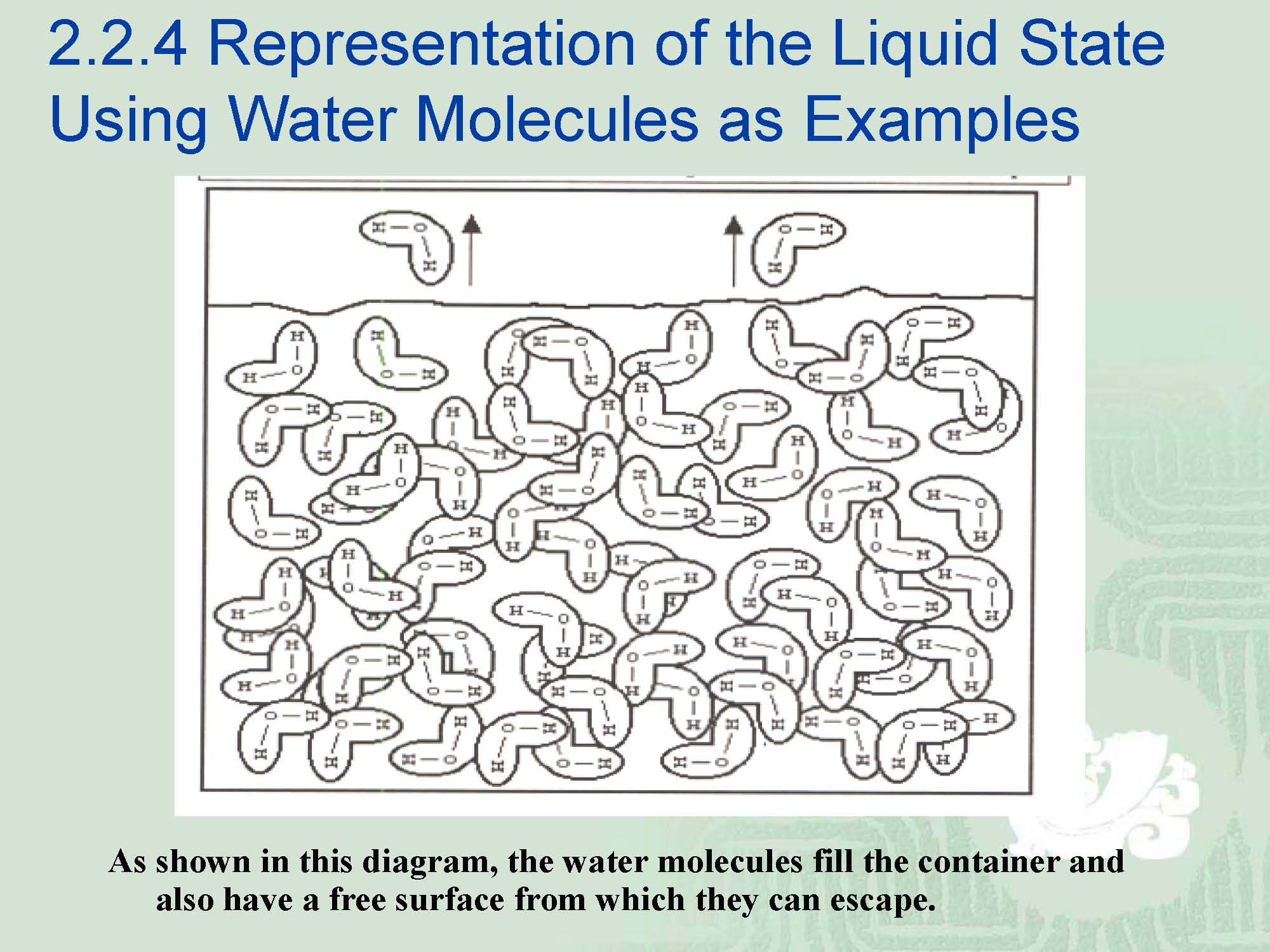
¡Thus,we conclude that the molecules of a liquid are free to slide past one another but the overall assemblage of molecules does not have a definitive form, except that of the container used to hold it.
¡Liquid definition—a substance or state of matter which has the capacity to flow under extremely small shear stresses to conform to the shape of any confining vessel, but is relatively incompressible and lacks the capacity to expand without limit.
¡Summary—As we change the state of matter, the translational degrees of freedom in liquids become severely restricted in relation to those of the gaseous state.
¡而且,对液体而言,振动和转动自由度也受到一定限制.
C. The Solid State
¡In a molecular sense, the solid state is that the molecules become ordered.
¡Another way to say is that they form a lattice-like framework.
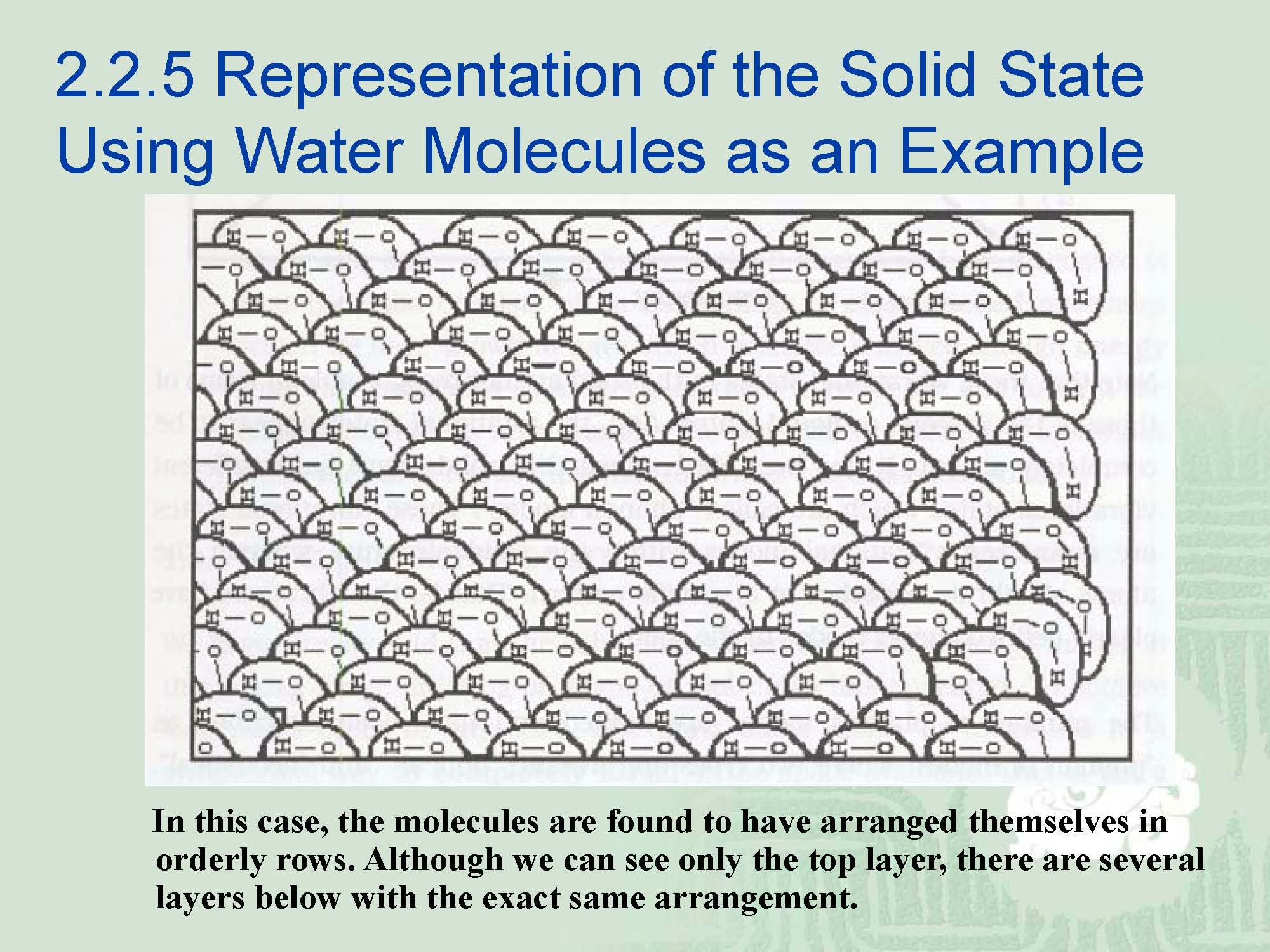
¡This is not the exact arrangement found in ice but is a stylized representation of the solid state of water.
¡It should be clear that as we change the state of matter, the translational degrees of freedom present in gases become restricted in liquids and disappear in solids.
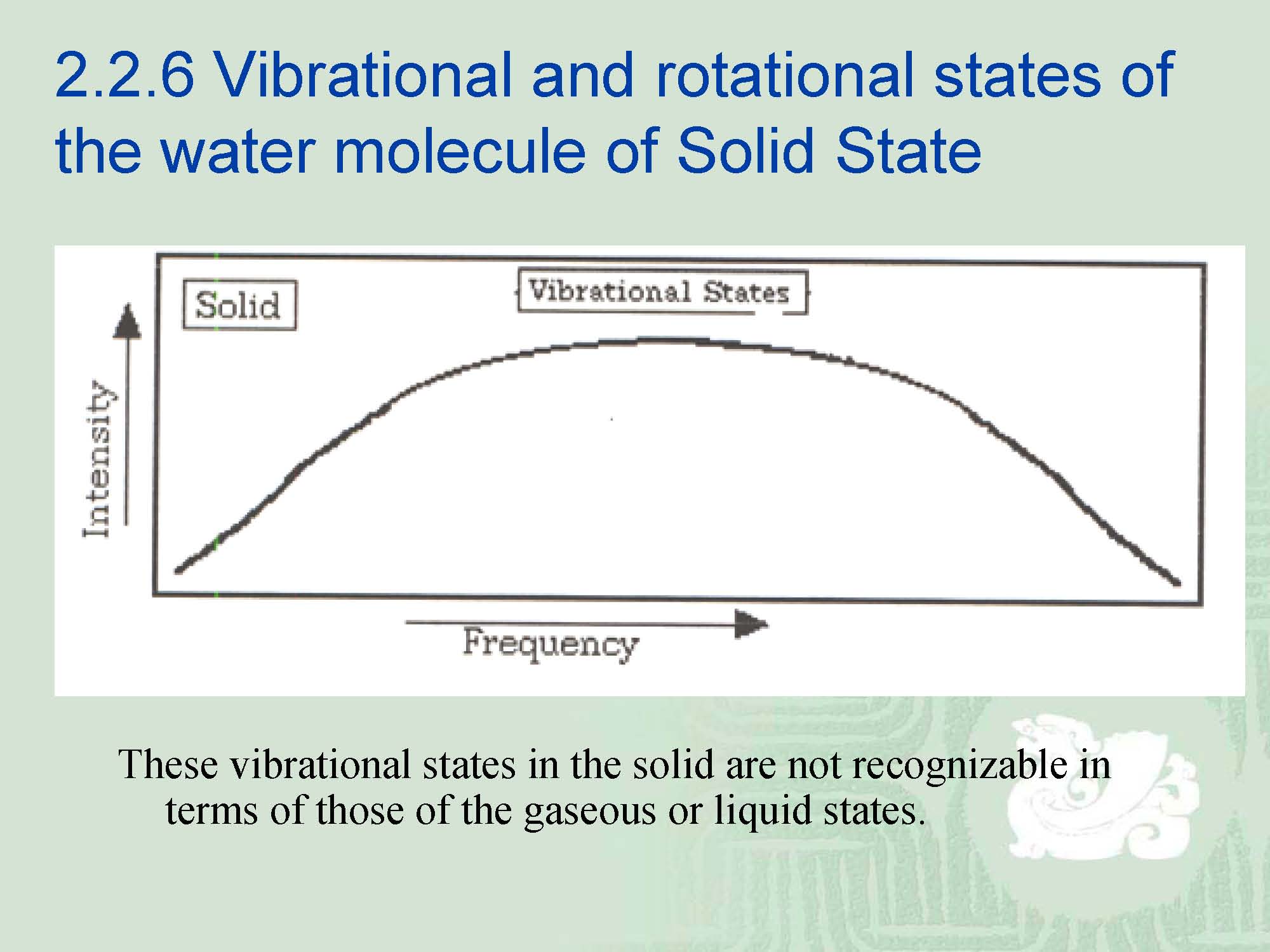
¡The rotational states appear to be completely absent. It has been determined that solids have quite different vibrational states which are called “phonon modes”.
¡That is, the vibrations have clearly defined energy modes in the solid.
2.2.7 Number of branchesof phonon dispersion
¡For the solid state, there will be a specific number of phonon branches found in the vibrational spectrum of any given solid, which depends upon the number of atoms composing the solids.
¡Acoustical=y atoms/molecule
¡Optical=3y-3
¡Phonon State: For water with 3 atoms per molecule
¡Acoustical=3
¡Optical=6
¡Phonon, 声子,点阵(晶格)振动能量的量子。它具有确定的能量和准动量。它的行为像一个粒子,所以它是准粒子。可以分为声学声子和光学声子,也可以分为横声子和纵声子。它的引入对处理有关晶格振动问题带来极大的方便。遵从玻色-爱因斯坦统计,在简谐近似下,声子可以按理想气体处理。而当光子、中子、电子等受到晶格作用时,看成它们与声子的碰撞作用来处理。
Summary of the majordifference
¡The major difference between the 3 phases we have discussed is that the solid consists of an assemblage of close-packed molecules which we have shown to have arisen when we removed enough energy from the molecules so as to cause them to condense and to form the solid state.


