2.1 Phase Changes of Solids, Liquids and Gases
--Introduction to how matter exists
¡Matter was originally defined as “anything that occupies space and has weight”.----18century
¡Background: Originally, in the 18th century,scientific revolution was getting started, sci. was referred to as “Natural Philosophy”.
--Three forms (phases) of matter exist
¡Earlyworkers knew that matter existed in three forms, i.e.-solids, liquids andgases, up to now, including plasma, too.
¡Changesof state—reversible transformations between the three forms of matter.----Phasechanges
¡Organicmaterials have a carbon backbone.
¡Nearlyall inorganics are solids at ambient temperature. At elevated temp., theytransform or melt to form a liquid andthen a gas.
¡Most of these phase changes are reversible.
¡We will be more interested in inorganics because of their greater therma lstability. Additionally, inorganic compounds have physical properties that cannot be duplicated in the organic domain of chemistry, and vice-versa.
--Phase Changes of Solids, Liquids and Gases
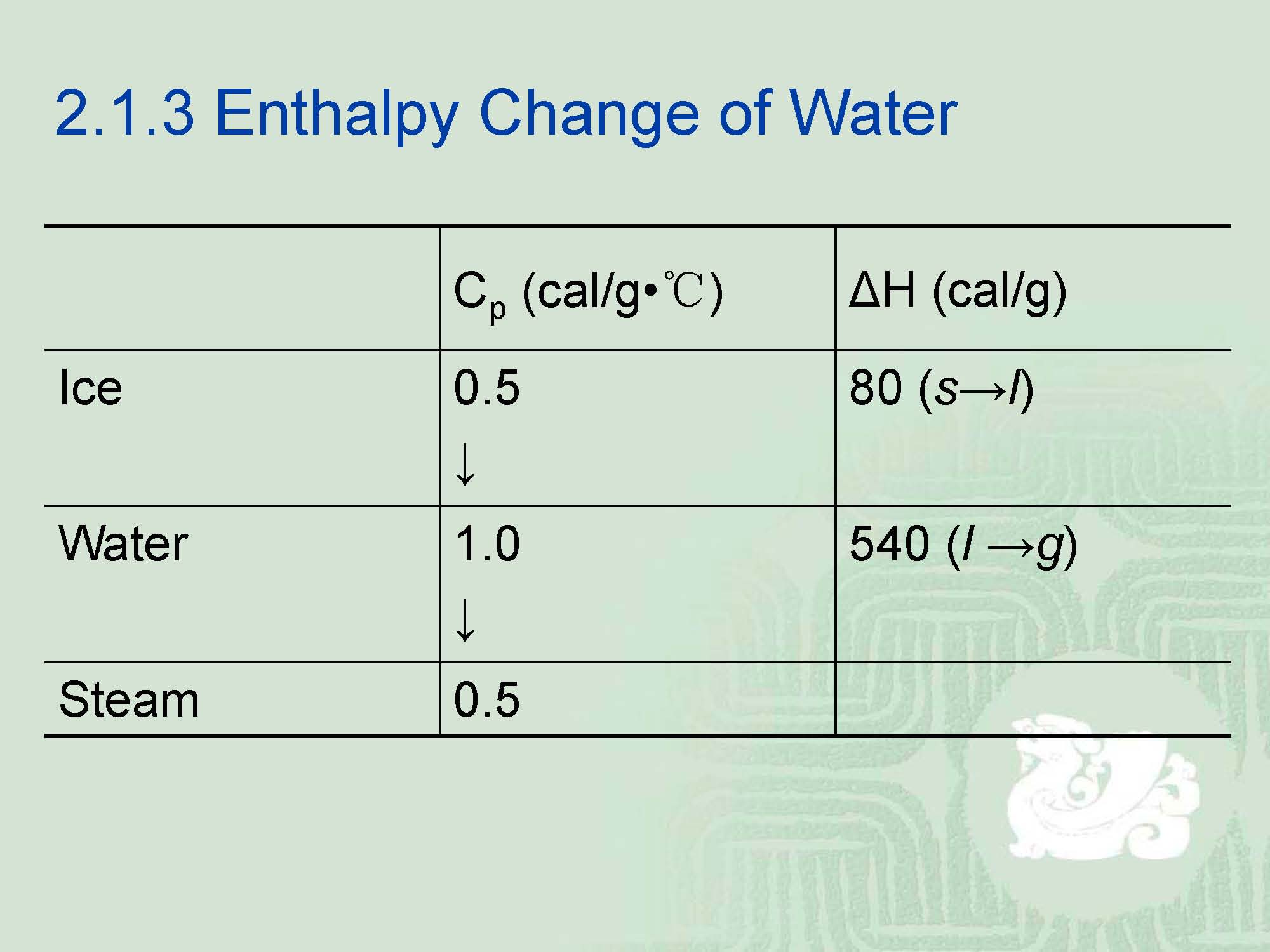
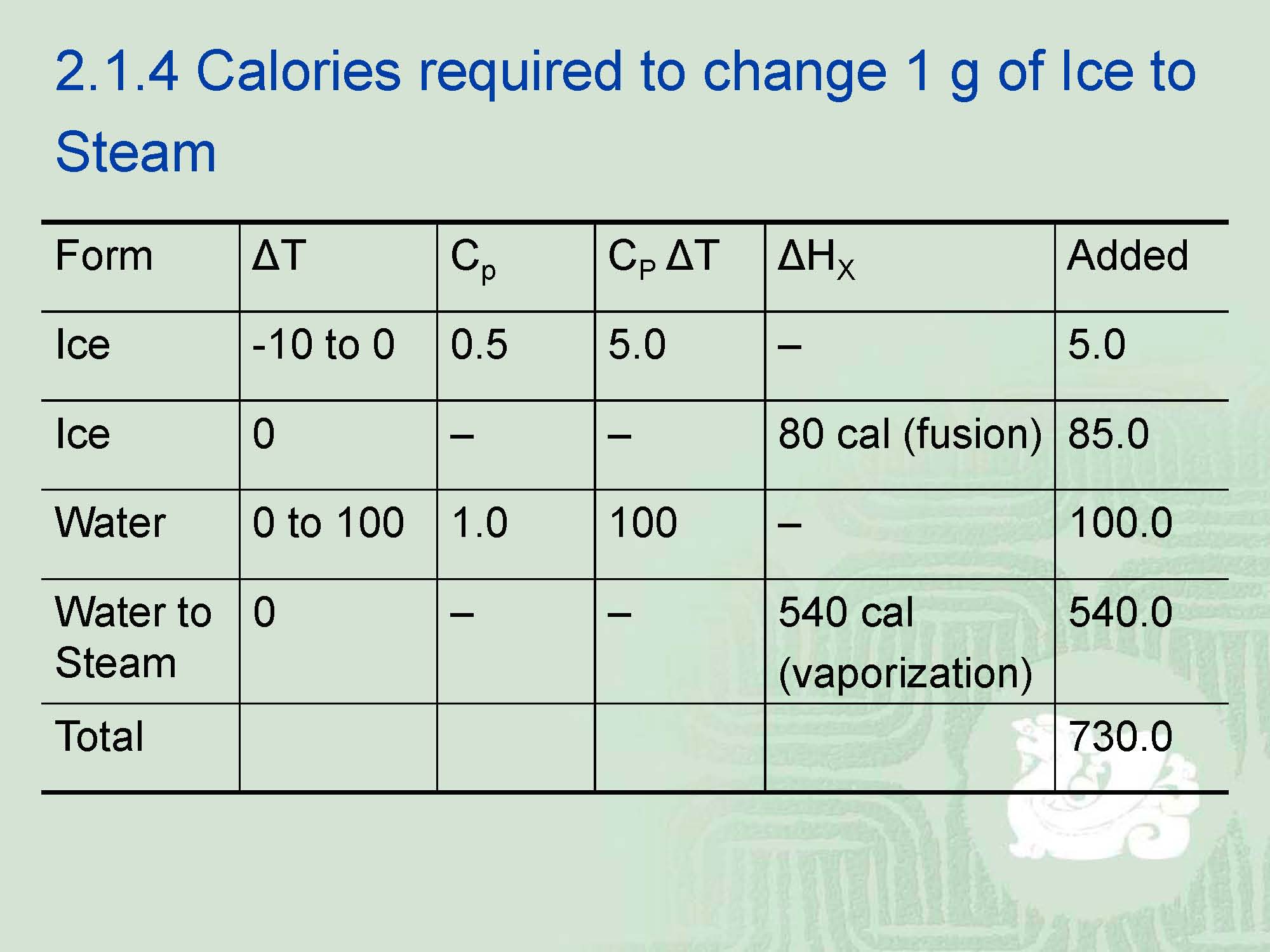
¡The best way to understand phase changes is to examine those observed with water,namely ice, water and steam.
¡The actual difference between them is a matter of energy.
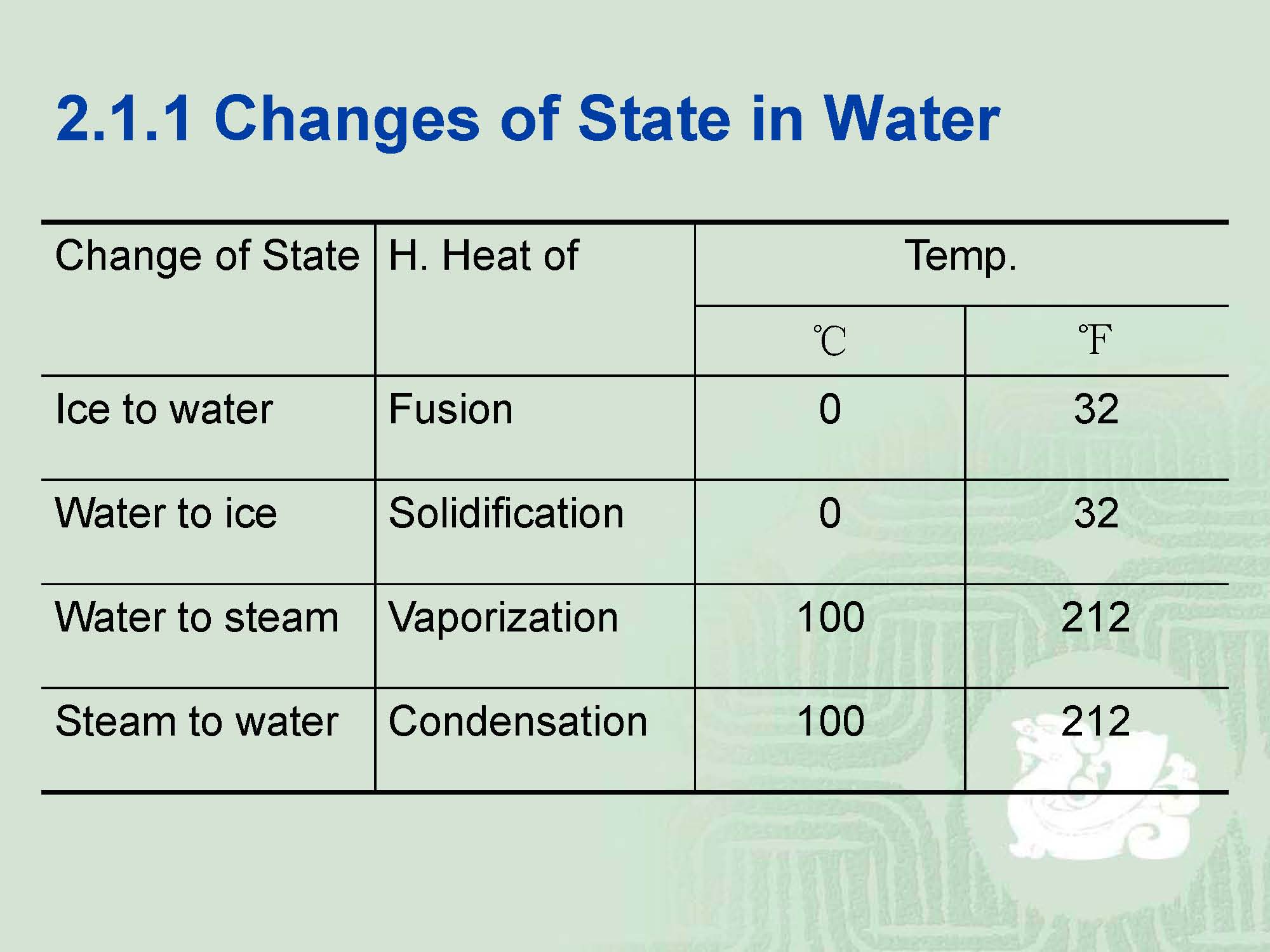
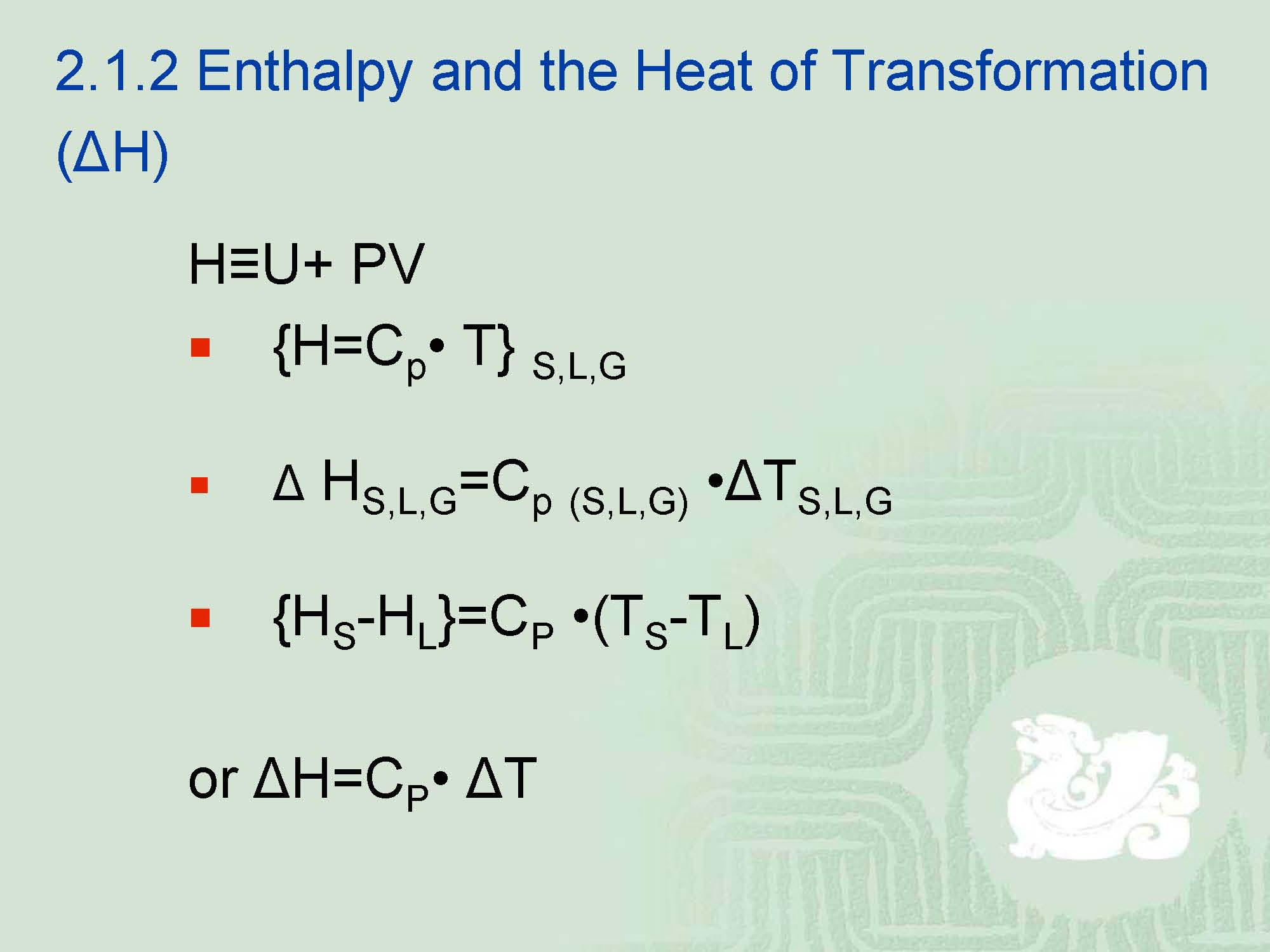
H—enthalpy—heat oftransformation
¡Heat capacity itself was originally defined as the amount of heat required to raise the temperature of one cubic centimeter (ml, cc.) of water (whose density was later defined as 0.9999 @ 3.98℃) by one degree.
¡However,after this concept had been defined, it was found that the same amount of heat did not raise the internal temperature of other materials to the same degree.----This led to the concept of heat capacity, heat transformation.
¡CP,meaning the thermal capacity at constant pressure, sometimes, it is also called specific heat, meaning the ratio of thermal capacity of any given materials to that of water, defined as 1.000.----This is the amount of heat in calories that it takes to raise any given material 1.00℃.
--CP—heat capacity in constant pressure
¡CV,the thermal capacity at constant volume, because most materials expand over a given temp. range, the measurement becomes complication. Its use is rare nowadays.
-- CV–heat capacity in constant volume