中文课件:1 水的特性与生物学意义.PPT
1. Properties of Water
Water is a polar molecule
Water, H2O, is a molecule with polarity. The central oxygen atom is more electronegative than the two hydrogen atoms, and so the electrons shared in the two bonds spend more time around the oxygen atom than around the hydrogen atoms. This structure is shown below:
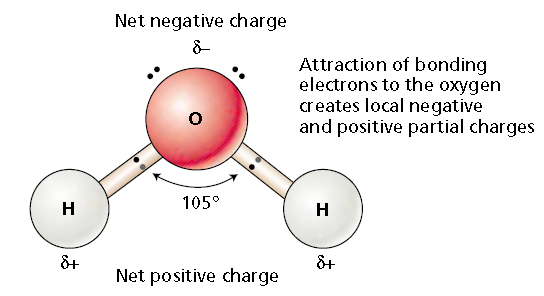
This electron distribution gives the central part of the water molecule a partial negative charge. The terminal hydrogen atoms thus have a partial positive charge. These partial charges of course mean that the hydrogen ends of one water molecule are attracted to the central oxygen portions of a neighboring water molecule. This kind of attraction is called hydrogen bonding:
Water is an excellent polar solvent
The polarity of the water molecule gives it the ability to dissolve polar molecules as well as less-polar molecules. In fact, among known liquids, water dissolves the widest range of chemical solutes. This makes water a medium for chemical transport and exchange. For plants, water dissolves soil minerals and carries them up the plant in the transpiration stream in the xylem; photosynthesis produces carbohydrates which are dissolved in water and carried from the leaf to the rest of the plant in the translocation stream in the phloem. Solubility in water causes phospholipids to orient themselves into membrane bilayers, and causes amino acid R-groups to twist in space to bring about protein conformation.
Water has high specific heat
The polarity of water and the resulting hydrogen bonding among water molecules means that it takes much heat (one calorie) to raise the temperature of 1 mL of water just 1° C. The hydrogen bonding has to be given a lot of energy to get them to vibrate and generate the temperature change. This property of water is called specific heat. It means that this liquid can absorb much heat from the various chemical reactions occuring in cells without temperature change; it is a heat buffer. It helps maintain an even plant body temperature.
Water has a high latent heat of vaporization
Among liquids, water has the highest latent heat of vaporization (44 kJ • mol-1) which is also known as heat of fusion. This means that when water goes from liquid to gas it takes a lot of energy. This property can obviously be traced directly to hydrogen bonding again. As the highest energy molecules in the liquid achieve what it takes to move away as a gas, their energy is removed from the liquid and it gets cooler. We sometimes refer to this as evaporative cooling. This is a critical property in maintaining the temperature of dark green leaves essentially "parked" in sunshine. A green car parked in the sunshine demonstrates the greenhouse effect; the leaf would do the same except that the water evaporating out of the stomata in the epidermis carries away the excess heat. The water is replaced by the transpiration stream in the xylem.
Water demonstrates adhesion and cohesion
The partial polarity of the water molecule makes it attractive to polar and less-polar surfaces. Water adheres to and climbs up materials like glass (forming a meniscus). The fact that water molecules attract each other makes them cohesive. These two properties allow water to climb up small-diameter tubes and remain in an unbroken fluid column; this is called capillarity. The column of water will climb inside the small tube to a height determined by:
rise in m = 14.9 • 10-6 m2 (radius in m)-1
Obviously water will climb higher in tubes of smaller radius that those of larger radius. In fact given a tracheid with a radius of 14.9 µm, the rise of water in that tracheid would be: 14.9 • 10-6 m2 (14.9 • 10-6 m)-1 = 1 meter! That of course is enough height to explain how water might get to the top of an herb in the xylem. It would fail to explain completely how water gets to the top of a 70 meter tall pine tree. We shall see later that in addition to capillary climb, the water is pulled through the xylem by evaporation. This evaporative pull is the major force in movement to the top of tall trees. This is accomplished by capillary movement of water away from the xylem along tiny intercellular spaces. The water bathing the mesophyll cells occupies even smaller spaces among the cellulose and hemicellulose polymers of the cell wall. These are in the range of 10-8m in radius. The evaporative pull is achieved in large part by capillarity of the very tiny cell-wall spaces.
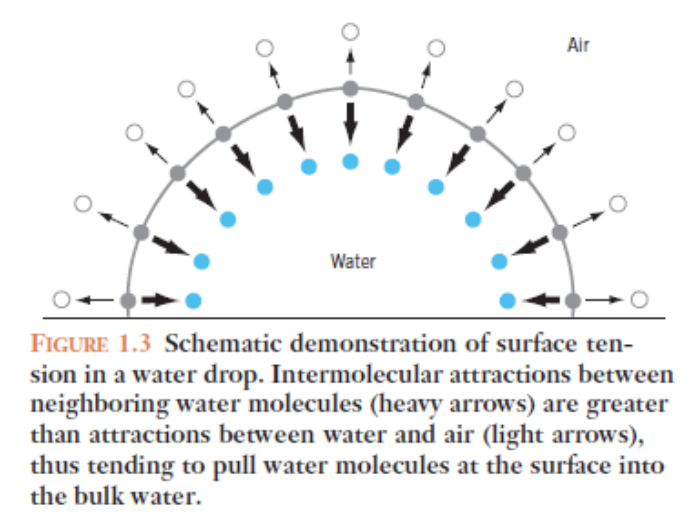
The cohesion of water molecules to each other relative to the much less polar N2 and O2 of air or other surfaces leads to the property called surface tension. This property is responsible for the beading-up of water on leaf epidermal waxes. The drop rounds up because of its cohesiveness and the lack of adhesion to the non-polar wax allows the drop to roll off of the leaf and to drip off, onto the soil below the leaf. This of course provides the water for the root to take up nutrients, and to cool that leaf ultimately by evaporative cooling.
Water has a high surface tension
It takes a lot of energy to break through the surface of water, because watermolecules at the surface are attracted (cohesion) to others within the liquidmuch more than they are to air. Thus, wateracts as though it has a skin.
Water has high tensile strength
The cohesive property of water keeps the column of water in the xylem unbroken all the way up to the top of a tree. A failure to do this would produce cavitation in the xylem and this would stop all flow of water up the tree in that column of xylem elements. In very small capillary tubes, the backwards-pull (tension) of the weight of the column of water below a given point may reach -30 MPa (megapascals) without breaking the column. This means that such a narrow column of water is about 1/10 as strong as copper or aluminum wire of similar diameter!
Water is not compressible
While gases can be compressed into smaller and smaller spaces, liquid water is not so compressible. Thus compression of water into a space surrounded by a cell wall produces turgor pressure. This form of hydraulic pressure is critical for cell growth, for the opening and closing of stomata, flow processes in translocation in the phloem, exchange of materials within and between cell compartments, and for the rigidity and support for herbaceous (not supported by lignin in wood) plants. Turgor keeps petals and leaves extended into the air and prevents wilting. Units of pressure are:
1 atmosphere = 14.7 lbs in-2 = 760 mm Hg = 1.013 bar = 0.1013 M Pa
typical tire pressure is 0.25 MPa.
Water is highly transparent
Visiblelight and UV can penetrate through water, thus submerged plants can survive;Furthermore, the inner mesophyll cells can absorb the penetrated light tophotosynthesize as well.
Water is an excellent solvent
The polarity of the water moleculegives it the ability to dissolve polar molecules as well as less-polar molecules. In fact, amongknown liquids, water dissolves the widest range of chemical solutes. This makes water an ideal medium for chemical transport and exchange.


