The Pre-test will be allowed to take after 10:20 am of Feb-18-2020, some explanations to this test are listed below,
1. Pre-test is designed to collect enough information of the background and level of chemistry you have before this course. The exact results will help us to make an effective teaching plan and adjustment for you. Every student has to take the pre-test although your score of pre-test will NOT be counted into your course grade.
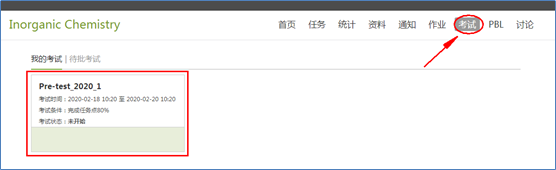
2. Prerequisites: You have to finish more than 80% of the tasks in chapter 1 before starting the pre-test.
3. Time limitation: 60 minutes, only once, please arrange your time and finish the pre-test as early as possible.
4. Content: There are 25 multiple-choices-with-one-answer questions and 3 short-answer question in pre-test.
5. Preparation: Good network connection and a computer are required for taking this test, smart phone is less convenient to finish the short-answer questions than laptop.
6. You can not see the results and score until all of your classmates finish the test and the teachers complete the analysis of that. The teaching group will inform you when it is ready.
7. For the computer user, the entrance of pre-test was showed in the picture below, while smart phone user can download the tutorial file (at the bottom of this page) to learn how to open pre-test in your mobile phone.
Don't worry about the pre-test, arrange your time and make a good preparation before taking it. You are able to finish the test smoothly! Good luck!