What is Aspirin?
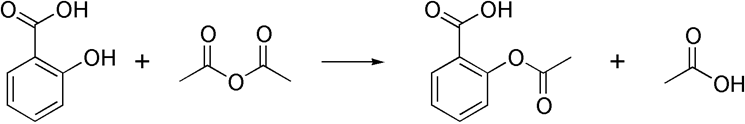
Aspirin is acetylsalicylic acid. It is prepared using salicylic acid (or 2-hydroxybenzoic acid ) reacting with acetic anhydride.
Aspirin is a commonly orally administered non-steroidal antiinflammatory drug for the treatment of pain and fever or inflammation due to various causes. It is sometimes used to treat or prevent heart attacks, strokes, and chest pain (angina). Aspirin should be used for cardiovascular conditions only under the supervision of a doctor. Acetylsalicylic acid binds to and acetylates serine residues in cyclooxygenases, resulting in decreased synthesis of prostaglandin, platelet aggregation, and inflammation. It works by reducing substances in the body that cause pain and fever and inflammation.
Aspirin is perhaps the most commonly used analgesic and antipyretic medication worldwide, having been in clinical use for over 100 years. This drug also inhibits platelet aggregation and is used in the prevention of blood clots stroke, and myocardial infarction (MI). Interestingly, the results of various studies have demonstrated that long-term use of acetylsalicylic acid may decrease the risk of various cancers, including colorectal, esophageal, breast, lung, prostate, liver and skin cancer. Aspirin is classified as a non-selective cyclooxygenase (COX) inhibitor and is available in many doses and forms, including chewable tablets, suppositories, extended release formulations, and others. Acetylsalicylic acid is a very common cause of accidental poisoning in young children. It should be kept out of reach from young children, toddlers, and infants. Aspirin can cause several forms of liver injury: in high doses, aspirin can cause moderate to marked serum aminotransferase elevations occasionally with jaundice or signs of liver dysfunction, and in lower doses in susceptible children with a febrile illness aspirin can lead to Reye syndrome.


