Unit 13 Glass Formation
Diezel introduced into the structural concepts of the alkali silicate glasses a new viewpoint, by considering the distribution of the alkalies. The structure of an alkali silicate glass is described as a random, continuous, three dimensional network. A distinction is made between two kinds of oxygen ions, the bridging oxygens and the non bridging oxygens. The addition of alkali oxides to silica leads to a breakage of a Si-O-Si chain. the alkali ions are placed near the broken bridge oxygen ions and are surrounded by a certain number of bridged oxygen ions. This allows a more or less random distribution of the non bridging oxygen ions among the bridging oxygen ions.
Sodium oxide or calcium oxide enter the holes in the network as ions. Fundamentally, glass becomes ionic in nature and this ionic nature gives it conduction at high temperature. However, at room temperature the ions are so restricted in their movements that glass is an insulater. With increasing thermal energy the number of bonds which can break increases. Thus, glasses have no specific melting point, but are characterized by a softening range.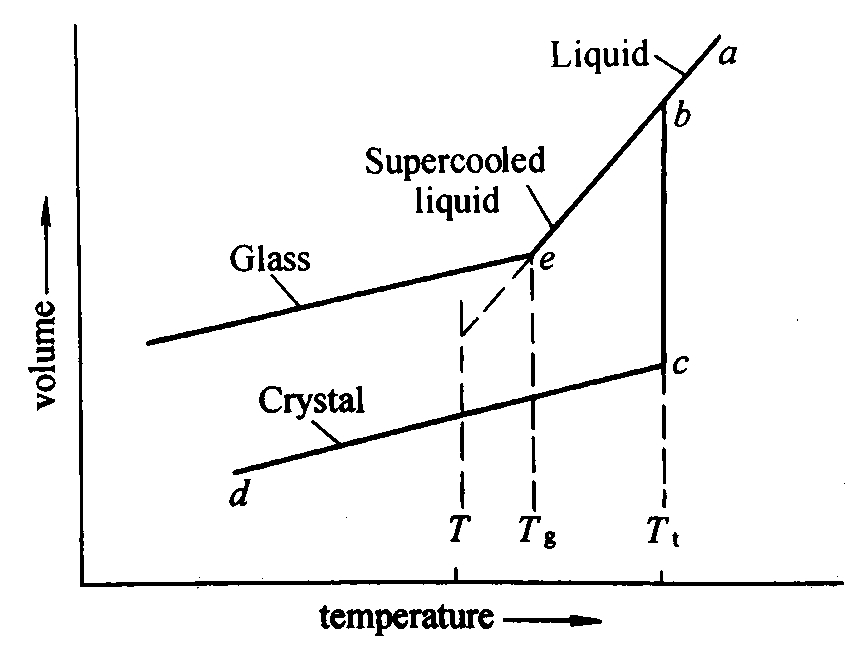
Fig. 3. 1 relation between the glassy, liquid and solid states (JONES, 1956)
The relationships between the liquid, the crystalline and the glassy states of a material are best explained in a volume temperature diagram for a glass forming substance shown in Fig.3.1. The volume of a substance decreases on cooling steadily along the line ab. At a sufficiently slow cooling rate with nuclei present in the melt, crystallization will take place at the temperature Tf accompanied by a decrease in volume following be. On further cooling, the crystalline substance contracts along cd. No crystallization will take place at Tf, if the rate of cooling is sufficiently rapid. The volume of the now supercooled liquid decreases on further cooling along the line be. At a certain temperature Tg, the volume temperature curve of the supercooled liquid undergoes a marked change in direction and continues almost parallel to the contraction curve of the crystalline form. The temperature Tg, at which the bend occurs, is called the transformation or glass transition temperature. Only below Tg it is correct to describe the substance as a glass. Between Tg and Tf the substance is a supercooled liquid. At Tg the viscosity is extremely high, about 10[12] Pa·s. If the temperature of the glass is held constant at T, the volume decreases slowly until it reaches a point on the dotted line. This process, by which the glass reaches a more stable condition, is known as stabilization. Other properties of the glass also change with time near the transition temperature. The properties of a glass depend to a certain extent on the rate at which it has been cooled, particularly through the temperature range near Tg. Also the exact value of Tg depends on the rate of cooling being lower the smaller the rate of cooling.
Alumina does not form a glass. When the phase diagram CaO-Al2O3 was determined in 1909, it was found that in a small composition range around the compound 12CaO-7Al2O3 the liquidus temperature drops to approximately 1400C and a glass formation was observed, having an Al2O3 composition range of 38%~65%. While pure calcium aluminate glasses have to be cooled rather quickly, their stability can be raised by adding about 5% SiO2. Glasses based upon calcium aluminate became of interest when the optical industry searched for glasses with good infrared transimissions.
For the production of technical glasses the ternary system Na2O-CaO-SiO2 is of great importance. The main composition of glasses belonging into this system covers the area of the NC3S6-βCS border. Devitrite, Na2O-3CaO-6SiO2, decomposes at 1045C forming wollastonite (CS) and a liquid. A homogeneous liquid is formed at 1325C. Devitrite is the primary phase which crystallizes form a large number of commercial glasses.
Thuringian glass enjoyed a particularly good reputation with respect to its workability. The sands found in this part of Germany contained some feldspar which introduced a few percent of alumina into the glass. Al2O3 suppresses the tendency of the glass to devitrify and improves its chemical resistance.
A soda lime silica glass of the compostition of devitrite has a liquidus temperature of 1325C, but some of its O[2-] ions are still mobile 700C below this temperature. The alkali ions are still mobile 1000C below the liquidus temperature. The alkali and alkaline earth ions do not seem to remain randomly distributed.
The following single oxides form glasses: SiO2, GeO2, B2O3, P2O5, As2O3,. Each of these oxides may be melted with a second oxide or mixture of oxides. Usually, however, there are limits to percentages of other oxides which may be added. In addition to the group of the five glass forming oxides, there exists a second group including TiO2, SeO2, MoO3, WO3, Bi2O3, Al2O3, Ga2O2, and V2O5 of which none will form a glass itself, but each will do so when melted with a suitable quantity of a second oxide or mixture of oxides. The oxides of the second group will be distinguished from the first single glass forming oxides by calling them: conditional glass forming oxides. A third group, called intermediate oxides, contains oxides such as ZnO, BeO, PbO, ZrO2, Sb2O3.
Selected from "Process Mineralogy of Ceramic Materials", W. Baumgart, A. C. Dunham, G. C. Amstutz, Heidelberg and Hull, 1984
Words and Expressions
bridging oxygen 桥(位)氧
nonbridging oxygen 非桥(位)氧
transition temperature 转变温度
liquidus 液相的
approximately 大约
calcium aluminate glass 钙铝酸盐玻璃
infrared 红外的
transmission 透射
ternary 三元的
devitrite 失透石
decompose 分解
thuringian 图林根州的


